Establishment method of release curve of rabeprazole sodium enteric-coated micro-pill capsule in acidic medium
A technology of rabeprazole sodium intestine and beprazole sodium intestine, which is applied in the direction of color/spectral property measurement, etc., can solve problems such as interference with measurement results, and achieve the effects of accurate establishment method, feasible operation and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
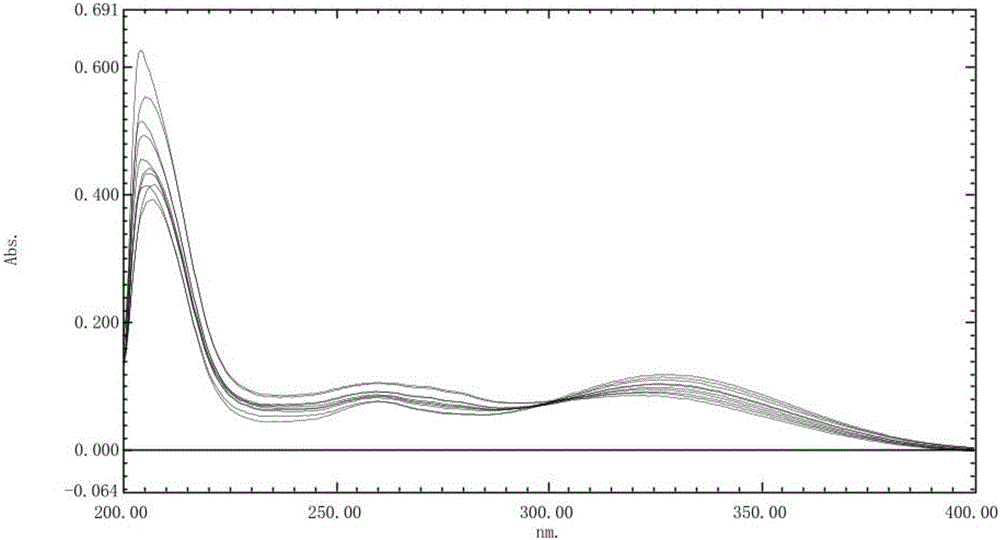
[0041] Accurately weigh 10 mg of rabeprazole sodium reference substance, add 0.1 mol / L hydrochloric acid solution to dissolve and dilute to make a solution containing 1 μg per 1 ml, put it in a water bath at 37.0 °C ± 0.5 °C, at 0, 30, 60, 90 1. At 120 minutes, take an appropriate amount of solution, cool to room temperature, and scan at a wavelength of 200-400 nm at different times according to UV-Vis spectrophotometry. The stability spectrogram of reference substance solution under 0.1mol / L hydrochloric acid solution sees figure 1 . The UV spectrum of each solution has a stable absorbance at 298nm.
Embodiment 2
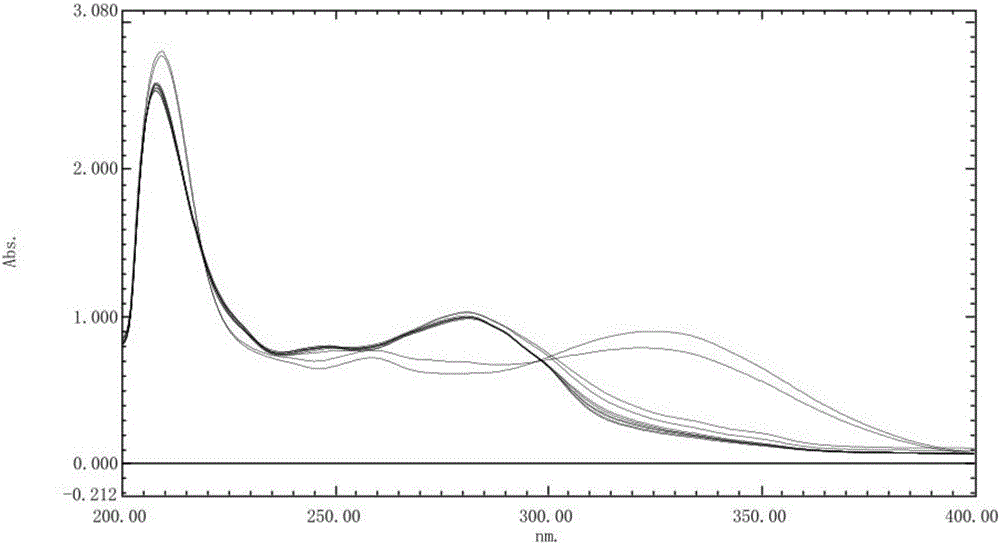
[0043] Accurately weigh 10 mg of rabeprazole sodium reference substance, add pH 6.0 phosphate buffer to dissolve and dilute to a solution containing 10 μg per 1 ml, put it in a water bath at 37.0°C±0.5°C, and set the temperature at 0, 30, 60, and 90°C respectively. 120 minutes, take an appropriate amount of solution, cool to room temperature, accurately measure 9ml and add 1ml 1mol / L hydrochloric acid solution, and scan at a wavelength of 200-400nm at different times according to ultraviolet-visible spectrophotometry. The stability spectrogram of the reference substance solution under the pH6.0 phosphate buffer solution is shown in figure 2 . figure 2 The first and second spectral lines at 298nm from top to bottom are the spectral lines of 0h and 1h at room temperature placed in a water bath at 37.0°C for 30 minutes, and the UV spectra of each solution are stable at 298nm except for 30 minutes at 37.0°C in a water bath of absorbance. Therefore, it is necessary to place the e...
Embodiment 3
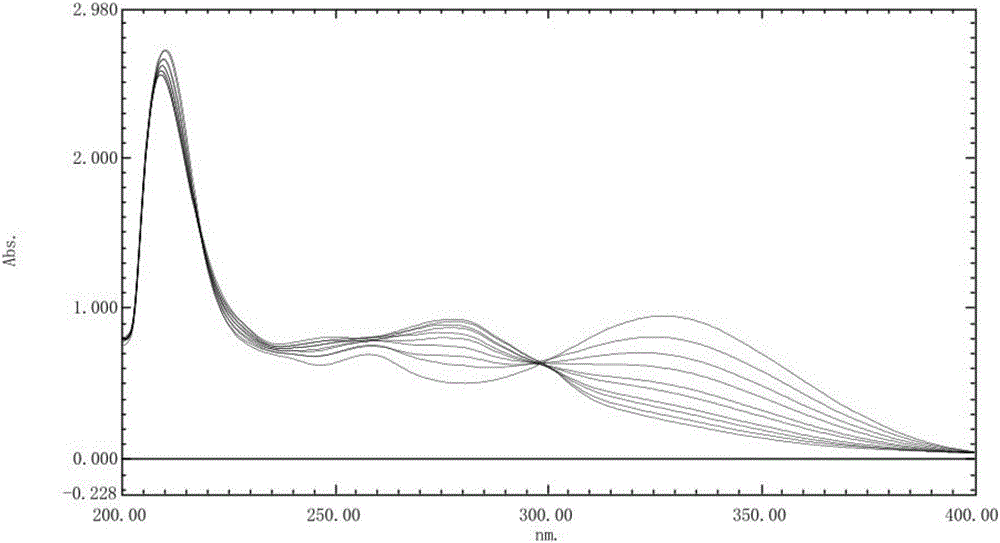
[0045] Accurately weigh 10mg of rabeprazole sodium reference substance, add pH6.8 phosphate buffer to dissolve and dilute to a solution containing 10μg per 1ml, put it in a water bath at 37.0°C ± 0.5°C, and set at 0, 30, 60, 90 120 minutes, take an appropriate amount of solution, cool to room temperature, accurately measure 9ml and add 1.2ml 1mol / L hydrochloric acid solution, and scan at a wavelength of 200-400nm at different times according to ultraviolet-visible spectrophotometry. The stability spectrogram of the reference substance solution under the pH6.8 phosphate buffer solution is shown in image 3 . The UV spectrum of each solution has a stable absorbance at 298nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com