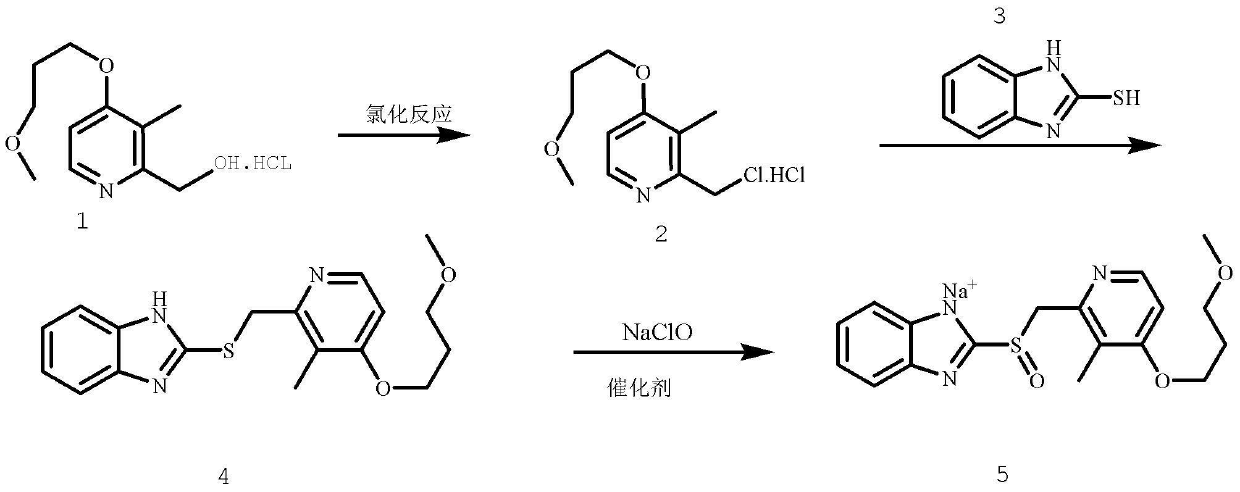
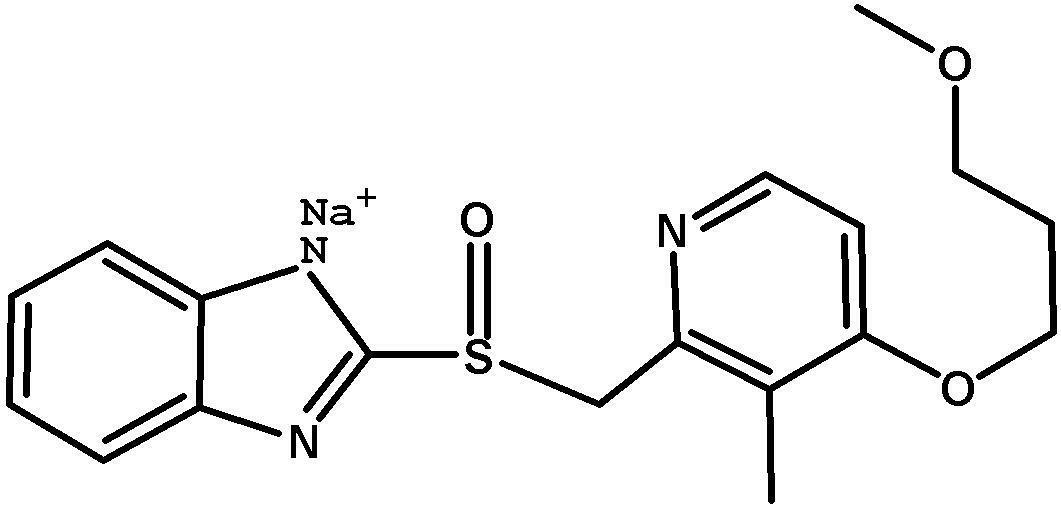
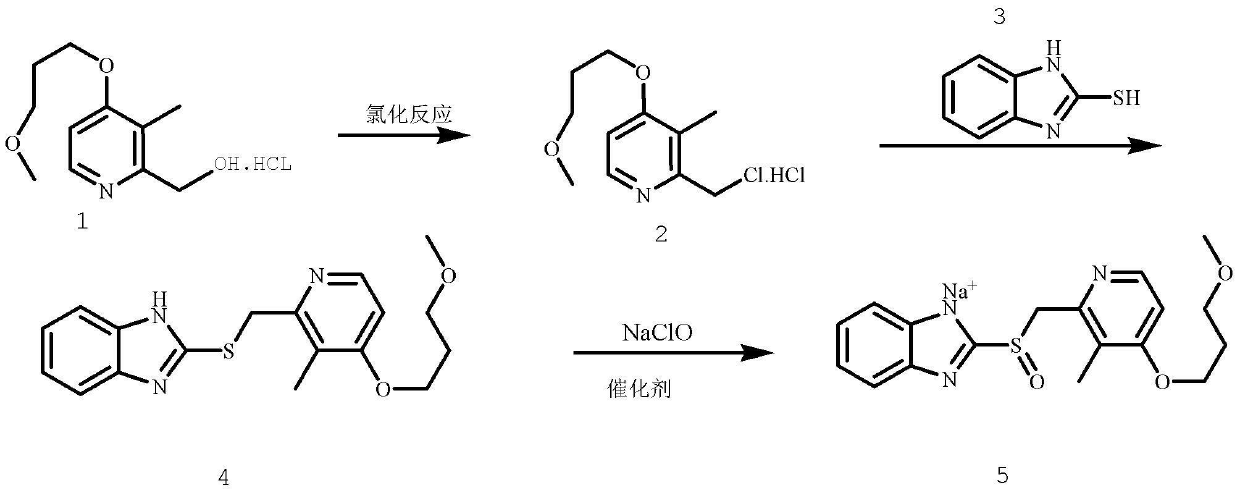
Method for pure water phase preparation of rabeprazole sodium
A technology of rabeprazole sodium and rabeprazole sulfide, applied in the field of medicine, can solve the problems of poor selectivity, long reaction steps and time, low reaction yield and the like, and achieves low cost, easy operation and product yield. high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Add 24.7g 2-hydroxymethyl-3-methyl-4-(3-methoxypropoxy)pyridine hydrochloride, 15.3g phosphorus oxychloride in reactor, stir, and reaction temperature remains on 45~ 55°C, reflux reaction for 0.5h, 24.2g of the product was obtained, the single-step yield was 91%, and the purity was 99% (HPLC);
Embodiment 2
[0033] Add 12.4g 2-hydroxymethyl-3-methyl-4-(3-methoxypropoxy)pyridine hydrochloride, 6.6g thionyl chloride in the reactor, stir, and the reaction temperature remains at 45~ 55°C, reflux reaction for 0.5h, 12g of the product was obtained, the single-step yield was 90%, and the purity was 99% (HPLC);
Embodiment 3
[0035] Add 12.4g 2-hydroxymethyl-3-methyl-4-(3-methoxypropoxy)pyridine hydrochloride, 6.6g thionyl chloride in the reactor, stir, and the reaction temperature remains at 0~ 30°C, reacted for 5 hours, and obtained 10.3 g of the product, with a single-step yield of 78% and a purity of 95% (HPLC);
[0036] The second condensation reaction:
[0037] Example 1
[0038] Add 15g of 2-mercaptobenzimidazole into the reactor, dissolve in aqueous sodium hydroxide solution (a small amount of acetone can also be added), pH=11~13, 0~55°C, stir, take the first step Example 1 The reaction solution was dripped slowly, kept dripping for 90 minutes, continued to react for 90 minutes, and filtered to obtain 34.3 g of the product, with a yield of more than 99%, a total yield of 90%, and a purity of more than 98% (HPLC).
[0039] Example 2
[0040] Add 7.5g of 2-mercaptobenzimidazole into the reactor, dissolve in aqueous sodium hydroxide solution (a small amount of acetone can also be added), pH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com