Rabeprazole sodium compound and novel preparation method thereof
A technology for rabeprazole sodium and compound is applied in the field of rabeprazole sodium compound and its new preparation method, which can solve the problems of long synthesis steps, unsuitable for industrialization, and difficult to control oxidation to sulfoxide and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
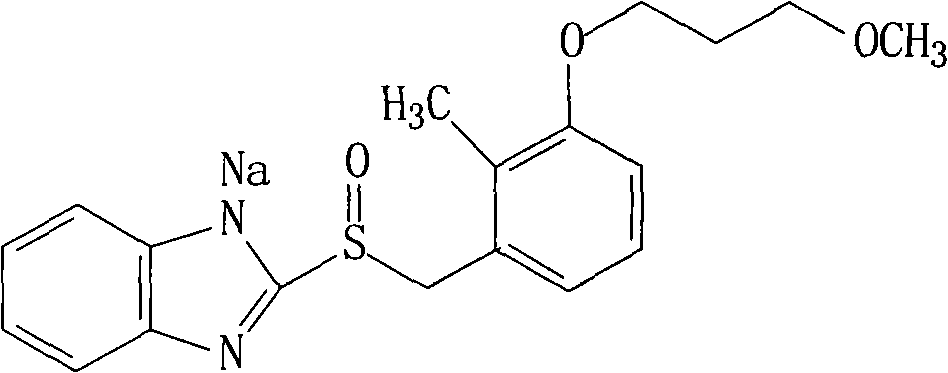
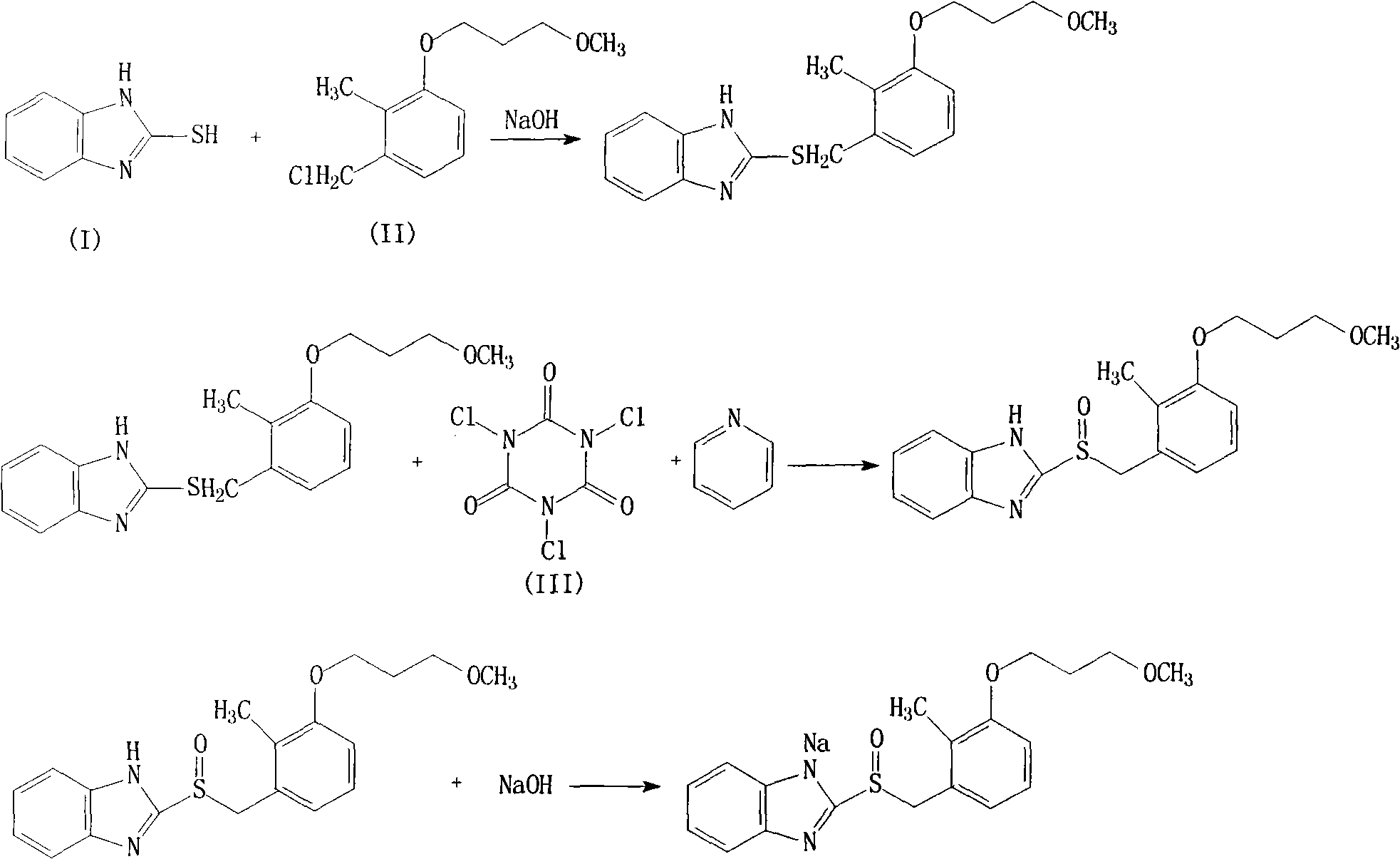
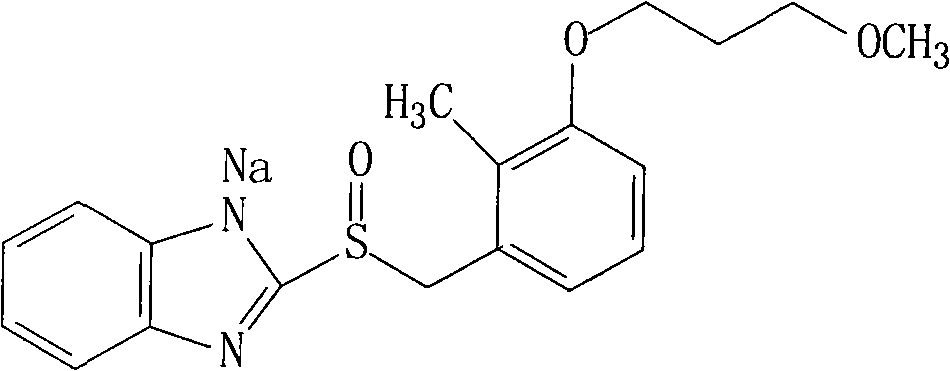
[0026] The synthesis of embodiment 1 rabeprazole sodium
[0027] (1) Synthesis of 2-{[4-(3-methoxypropoxy)-3-methylpyridin-2-yl]methylthio}-1H-benzimidazole
[0028] 300g (2mol) 2-mercaptobenzimidazole, 505g (2.2mol) 2-chloromethyl-3-methyl-4-[(3-methoxy)propoxy]-pyridine and 100g (2.4mol) Sodium hydroxide was added to 2500ml of ethanol, stirred and reacted at 50°C for 3 hours, then the ethanol was distilled off under reduced pressure, 5L of purified water was added, stirred, a solid was precipitated, filtered, and dried to obtain 632g of the product, with a yield of 92%.
[0029] (2) Synthesis of Rabeprazole
[0030] In a 5L reaction flask, add 343g (1mol) 2-{[4-(3-methoxypropoxy)-3-methylpyridin-2-yl]methylthio}-1H-benzimidazole, 2L The volume ratio is a mixed solvent of acetonitrile and dichloromethane of 1:1, then add 255g (1.1mol) trichloroisocyanuric acid and 95g (1.2mol) pyridine, stir at room temperature for 4 hours, then pour the reactant into 1L Stir in water, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com