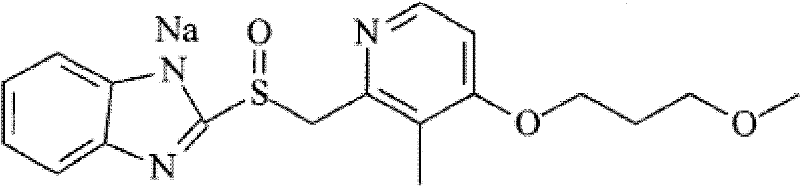
Rabeprazole sodium crystal compound and preparing method thereof
A technology for rabeprazole sodium and crystalline compounds, applied in the field of rabeprazole sodium crystalline compounds and their preparation, can solve the problems of low binding strength, difference in solubility of crystalline drugs, large free energy per unit surface and the like, and achieve quality assurance , good stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] [Example 1] Preparation of Rabeprazole Sodium Crystal Compound
[0038] 1) Add 60 g of the raw material drug rabeprazole sodium to 300 ml of water, stir and dissolve to obtain an aqueous solution of rabeprazole sodium;
[0039] 2) Add 480ml of a mixed solution of tetrahydrofuran and methanol (the volume ratio of tetrahydrofuran to methanol is 3:1) to the above aqueous solution of rabeprazole sodium under stirring at a speed of 300r / min. The activated carbon is kept decolorized at 25°C and filtered. Get filtrate
[0040] 3) The filtrate was dropped into 1440 ml of acetonitrile, cooled to 5° C., crystals were precipitated, filtered, and dried in vacuum to obtain 54.1 g of white rabeprazole sodium crystalline compound.
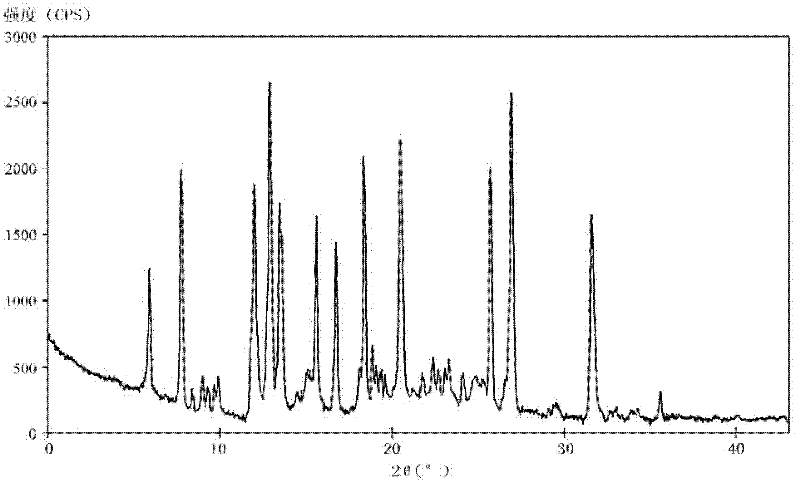
[0041] The melting point of the crystalline compound is 162-163°C detected by capillary method.
[0042] Using the PE 2400II elemental analyzer from Perkin-Elmer, USA, the elemental analysis (%) is: actual measured value (calculated value), C: 56.68 (56.68), H: 5.2...
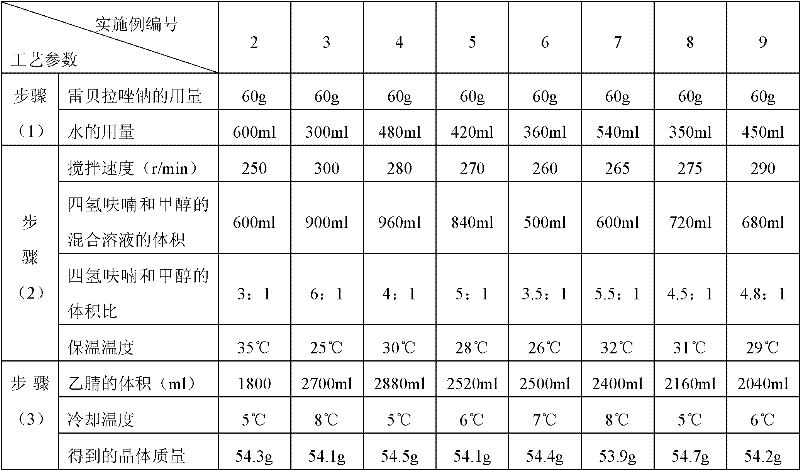
Embodiment 2-9
[0046]
[0047] The melting point of the crystalline compound obtained in Examples 2-9 was detected by capillary method to be 162-163° C., and elemental analysis was performed with the PE2400II elemental analyzer of Perkin-Elmer Company, USA. The result was similar to that of Example 1.
[0048] At the same time, the crystalline compounds of Examples 2-9 were measured by powder X-ray diffraction measurement, and the X-ray powder diffraction pattern represented by the diffraction angle of 2θ±0.2° was similar to that of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com