Rabeprazole sodium powder injection and preparation method thereof
A technology of rabeprazole sodium and powder injection, which is applied in the field of pharmaceutical preparations, can solve the problems of stability and related substances that have not been tested in experiments, and achieve good stability effects, good stability, and unique process effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Prescription of Rabeprazole Sodium Powder Injection:
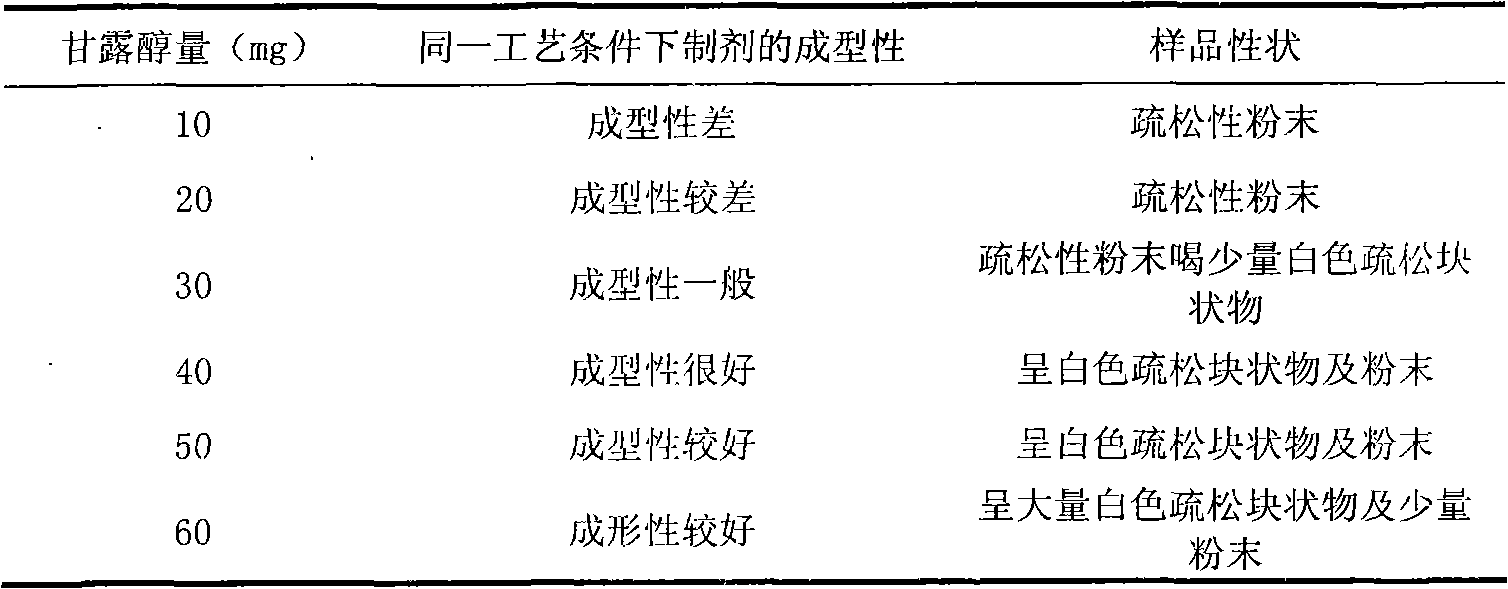
[0038] Rabeprazole Sodium 20g
[0039] Mannitol 40g
[0040] Appropriate amount of sodium hydroxide
[0041] The preparation method is:
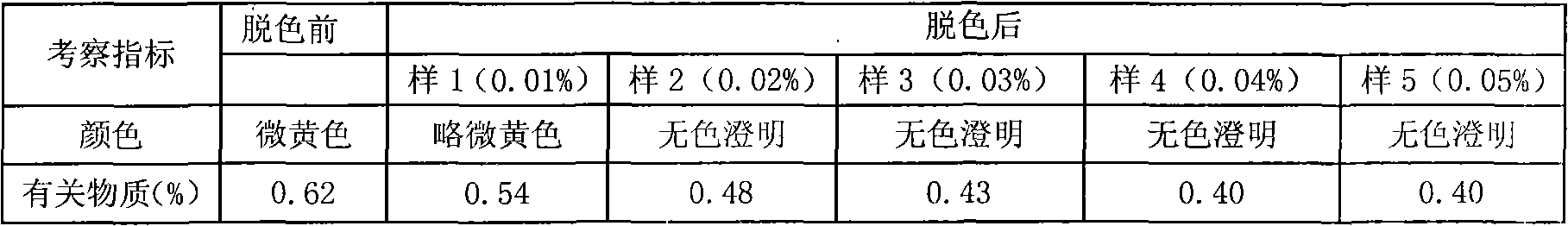
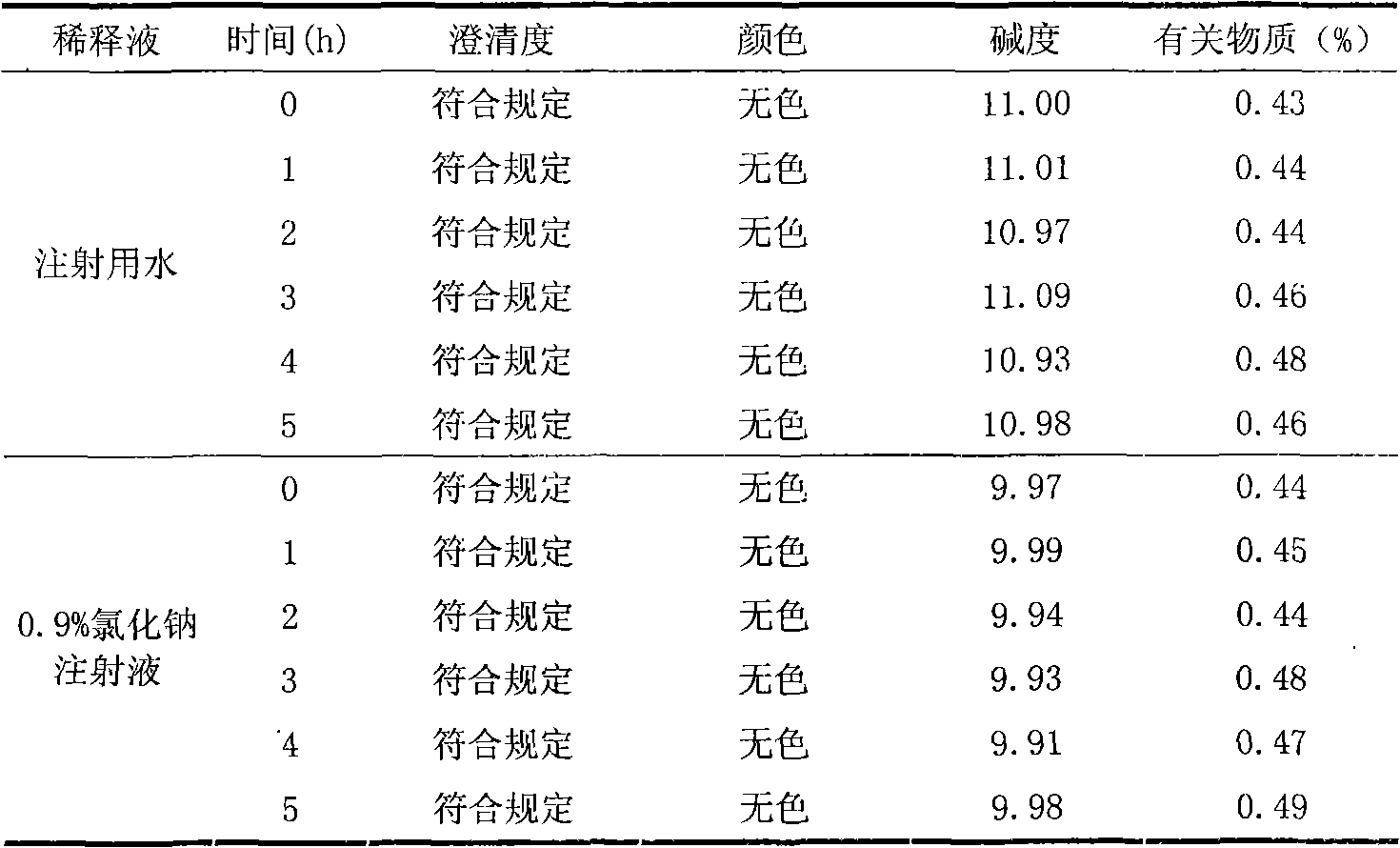
[0042] 1) Accurately weigh the prescribed amount of rabeprazole sodium and mannitol into a sterile container, add 1800ml of water for injection, stir to dissolve, and adjust the pH value of the solution to 10.5-11.5 with 0.1mol / L sodium hydroxide solution, Stir, add water for injection to 2000ml, then add 0.02%-0.04% activated carbon, stir for 30 minutes, and decarbonize. Filter through a 0.22 μm microporous membrane. Measure the solution content, determine the filling volume, and fill. A total of 1000 bottles were made.
[0043] 2) Freeze drying.
[0044] The sample is pre-frozen at -40°C for 9-11 hours; vacuumize, keep the vacuum at 10-30Pa, slowly raise the temperature to -20°C-25°C, and keep it for about 10-12 hours; then slowly raise the temperature to 30°C-35°C, Keep...
Embodiment 2
[0046] Prescription of Rabeprazole Sodium Powder Injection:
[0047] Rabeprazole Sodium 20g
[0048] Mannitol 40g
[0049] Appropriate amount of sodium hydroxide
[0050] Preferably, the preparation method of powder injection of the present invention is:
[0051] 1) Accurately weigh the prescribed amount of rabeprazole sodium and mannitol in a sterile container, add 1800ml of water for injection, stir to dissolve, adjust the pH value of the solution to 11 with 0.1mol / L sodium hydroxide solution, stir, Add water for injection to 2000ml, then add 0.03% activated carbon, stir for 30 minutes, and decarbonize. Filter through a 0.22 μm microporous membrane. Measure the solution content, determine the filling volume, and fill. A total of 1000 bottles were filled.
[0052] 2) Freeze drying.
[0053]The samples were pre-frozen at -40°C for 10 hours; evacuated and kept at 20 Pa, slowly raised to -20°C and kept for about 10 hours; then slowly raised to 30°C and kept for 6 hours, t...
Embodiment 3
[0055] Prescription of Rabeprazole Sodium Powder Injection:
[0056] Rabeprazole Sodium 20g
[0057] Mannitol 45g
[0058] Appropriate amount of sodium hydroxide
[0059] Preparation method is the same as embodiment 2
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com