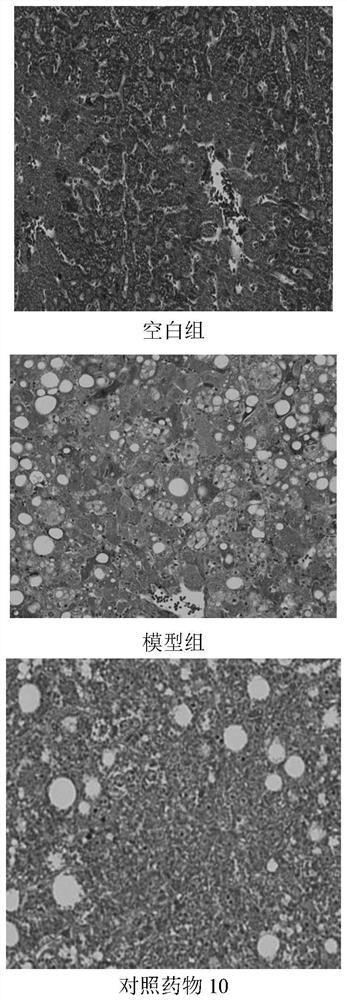
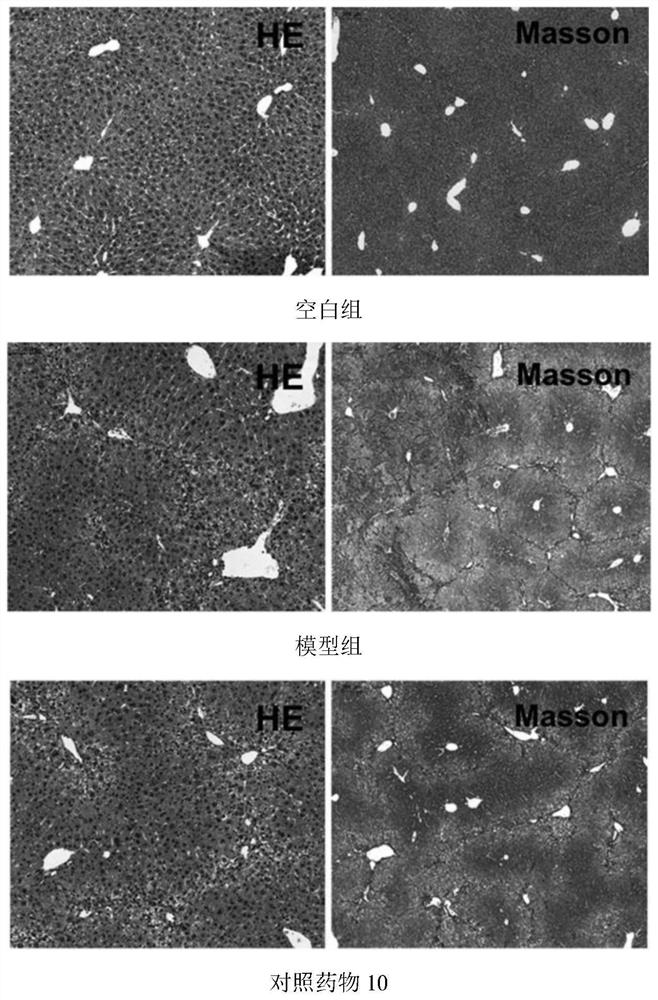
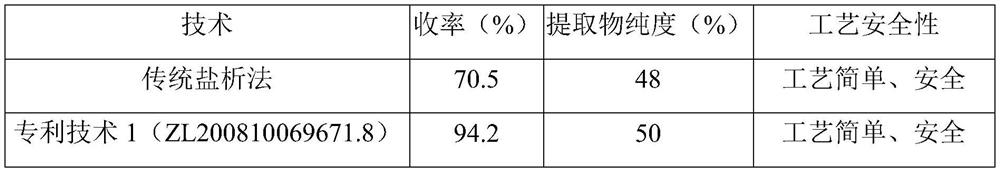
Application of Xianglian product or combination of Xianglian product and antibiotics in anti-helicobacter pylori drugs
A technology of Helicobacter pylori and its products, which is applied in antibacterial drugs, medical preparations containing active ingredients, plant/algae/fungus/moss components, etc., which can solve the problems of poor safety and achieve high safety and simple preparation process , Improve the effect of antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Weigh 80 g of Yuhuanglian, extract by percolation with 0.5% sulfuric acid solution, until the color of the percolation liquid is very light (the total alkaloids in the extracted raw materials are more than 95% according to HPLC analysis); the percolation liquid is neutralized to pH=3.0 with calcium hydroxide , filtered; the filtrate was concentrated under reduced pressure to 1g Yuhuanglian / mL, cooled, and filtered; 10% sodium chloride was added to the filtrate, and stood at 5°C for 24 hours, precipitated and filtered to obtain extract Ⅰ; the filtered mother liquor was added 10% sodium chloride, and 0.5% zinc chloride, stand still at 5°C for 5 hours, precipitate and filter to obtain extract II; combine extract I and extract II precipitate to obtain Yuhuanglian extract.
[0045] Weigh 20g of Muxiang, extract with 5 times of water reflux for 1 hour, and collect volatile oil; filter the extracted aqueous solution, and then reflux the filtered residue with an appropriate amou...
Embodiment 2
[0048] Weigh 80 g of Yuhuanglian, extract by percolation with 2% sulfuric acid solution, until the color of the percolate is very light (the total alkaloids in the extracted raw materials are more than 95% according to HPLC analysis); the percolate is neutralized to pH=7.0 with calcium hydroxide , filtered; the filtrate was concentrated under reduced pressure to 2g Yuhuanglian / mL, cooled, and filtered; 1% potassium bromide was added to the filtrate, and stood still for 96 hours at 25°C, precipitated and filtered to obtain extract Ⅰ; the filtered mother liquor was added 20% potassium bromide, and 0.1% ferric bromide, stand at 25°C for 1 hour, precipitate and filter to obtain extract II; combine extract I and extract II precipitate to obtain Yuhuanglian extract.
[0049] Weigh 20g of Muxiang, extract with 5 times of water reflux for 1 hour, and collect volatile oil; filter the extracted aqueous solution, and then reflux the filtered residue with an appropriate amount of water to ...
Embodiment 3
[0052] Weigh 80 g of Yuhuanglian, extract by percolation with 1% sulfuric acid solution, until the color of the percolate is very light (the total alkaloids in the extracted raw materials are more than 95% according to HPLC analysis); the percolate is neutralized to pH=5.0 with calcium hydroxide , filtered; the filtrate was concentrated under reduced pressure to 1g Yuhuanglian / mL, cooled, and filtered; 10% sodium iodide was added to the filtrate, and stood at 10°C for 48 hours, precipitated and filtered to obtain extract Ⅰ; the filtered mother liquor was added 10% sodium iodide, and 0.5% magnesium iodide, stand still at 10°C for 10 hours, precipitate and filter to obtain extract II; combine extract I and extract II precipitate to obtain Yuhuanglian extract.
[0053] Weigh 20g of Muxiang, extract with 5 times of water reflux for 1 hour, and collect volatile oil; filter the extracted aqueous solution, and then reflux the filtered residue with an appropriate amount of water to ext...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com