Long-acting glucagon-like peptide-1 analogue dimer and application thereof
A technology of glucagon and analogues, applied in the application of drugs, in the field of dimer of glucagon-like peptide-1 analogues, can solve the problems of drug compliance that needs to be improved, and achieve the improvement of clinical application compliance , Avoid safety risks, hypoglycemic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] A. Preparation of GLP-1 analogue monomer:
[0063] 1) Synthesis: Fmoc method is adopted, and the step-by-step synthesis method is implemented according to the following steps:
[0064] a) Fmoc-Gly-resin is obtained by coupling a resin solid phase support and Fmoc-protected glycine in the presence of an activator system;
[0065] b) Connect amino acids in accordance with the amino acid sequence of the peptide sequence by solid-phase synthesis to obtain a peptide-resin conjugate with N-terminal Fmoc-protection and side chain protection; take the following protection measures for amino acids with side chains: use Boc for tryptophan, gluten Use OtBu for amino acid, Trt for glutamine for lysine, tBu for tyrosine, Trt or tBu for serine, OtBu for aspartic acid, tBu for threonine, and Trt or Boc for histidine.
[0066] c) cleavage, remove the protecting group and resin simultaneously, obtain the crude product of GLP-1 analogue monomer;
[0067] 2) Purification: Dissolve the c...
Embodiment 2
[0088] Preparation of N-terminal acylated GLP-1 analogue dimers:
[0089] 1) According to the monomer synthesis method in Example 1, a peptide-resin conjugate with N-terminal Fmoc-protection and side chain protection was synthesized;
[0090] 2) Remove the N-terminal Fmoc protecting group by conventional methods, suspend the resin-peptide conjugate in an appropriate amount of pyridine, add acetic anhydride or trifluoroacetic anhydride in an appropriate molar ratio, mix well, and place it to obtain the N-terminal histidine acyl Peptide-resin conjugates;
[0091] 3) Cleavage according to the method in Example 1 to obtain the crude peptide, purify, and lyophilize to obtain the monomeric target peptide;
[0092] 4) Prepare the dimer according to the method of Example 1.
[0093] The GLP-1 analog dimer formed by the following sequence monomers was prepared according to the above method:
[0094] GLP-1 analog dimer 3-3 formed by SEQ ID NO 3 and SEQ ID NO 3,
[0095] GLP-1 analog...
Embodiment 3
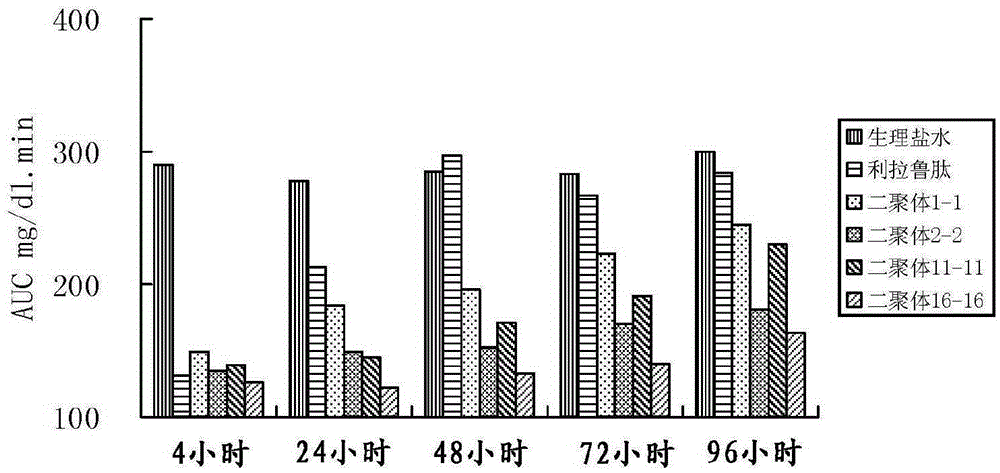
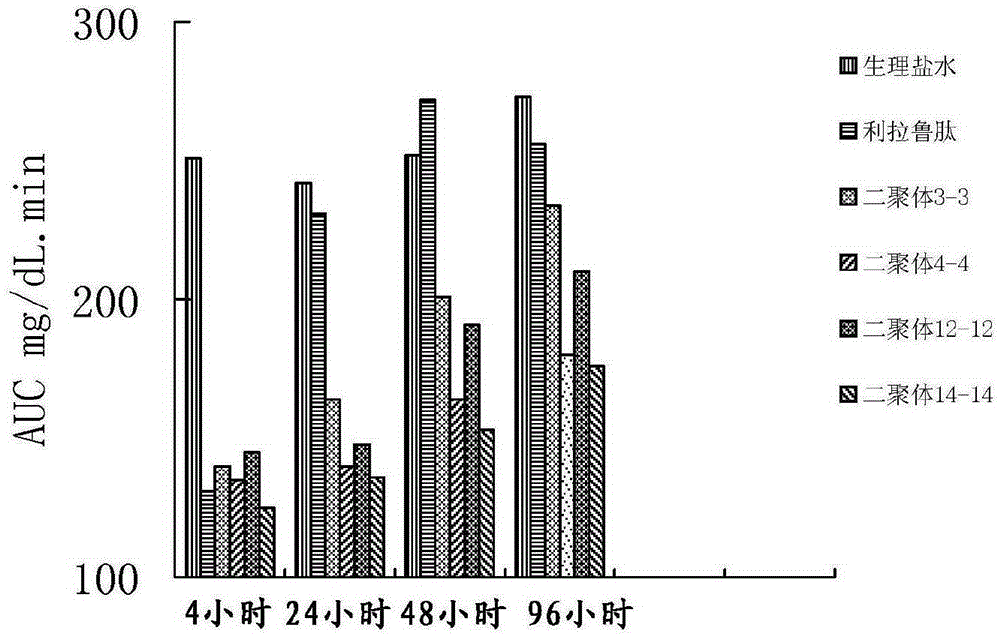
[0103] Evaluation of hypoglycemic effect of GLP-1 analog dimers 1-1, 2-2, 11-11, 16-16.
[0104] The glucose tolerance test of normal mice was used to evaluate the hypoglycemic effect of the GLP-1 analog dimer of the present invention. Methods: 30 normal mice (purchased from Shanghai Experimental Animal Center, Chinese Academy of Sciences) were randomly divided into 6 groups (blank control group, positive control group, and test group), with 5 mice in each group; The product (≥98%) was dissolved in physiological saline, and prepared as a 0.1 mg / ml sample solution. The mice in the test group were subcutaneously injected with 200 μl of the sample solution; the mice in the positive control group were injected subcutaneously with 20 μg liraglutide; the mice in the blank control group were injected subcutaneously with 200 μl saline. Glucose tolerance was measured at 4, 24, 48, 72, and 120 hours after injection. Glucose tolerance test: Orally administer glucose 2g / kg, measure the bl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com