Glucagon-like peptide-1 (GLP-1) analogs, and preparation method and application thereof
A technology for glucagon and analogues, which is applied in the field of application of glucagon-like peptide-1 analogues and their dimers, medicines, and can solve problems such as medication compliance that needs to be improved to achieve improved clinical application Compliance, avoiding safety risks, overcoming short half-life effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
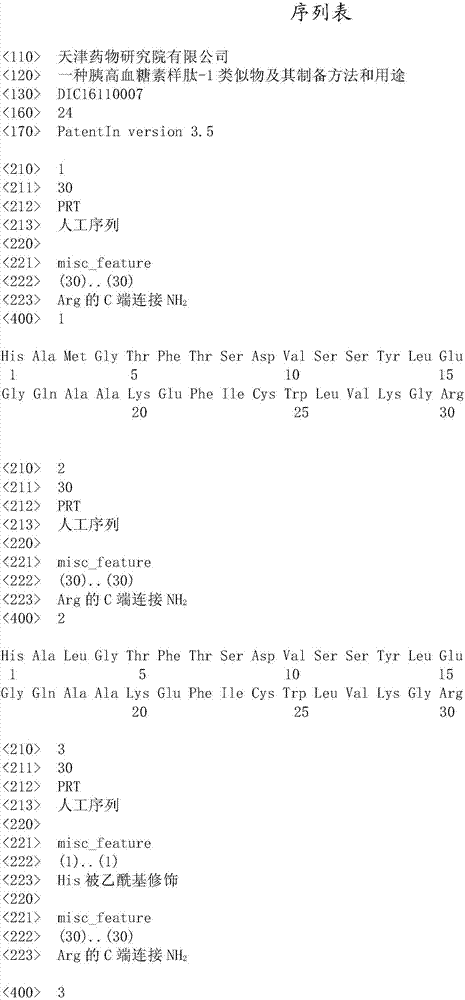
[0053] Example 1 Preparation of GLP-1 Analog Monomers and Dimers
[0054] A. Preparation of GLP-1 analogue monomer:
[0055] 1) synthesis: adopt Fmoc method, carry out synthesis method step by step according to the following steps:
[0056] a) Fmoc-Arg-resin is obtained by coupling an amino resin solid-phase support and Fmoc-protected arginine in the presence of an activator system;
[0057] b) Connect amino acids in accordance with the amino acid sequence of the peptide sequence by solid-phase synthesis to obtain N-terminal Fmoc-protected and side chain-protected peptide-resin conjugates; amino acids with side chains are protected as follows: tryptophan with tert-butoxy Carbonyl (Boc), glutamic acid tert-butyl (OtBu), lysine tert-butoxycarbonyl (Boc), glutamine trityl (Trt), tyrosine tert-butyl (tBu ), trityl (Trt) or tert-butyl (tBu) for serine, oxygen-tert-butyl (OtBu) for aspartic acid, tert-butyl (tBu) for threonine, trityl for histidine group (Trt) or tert-butoxycar...
Embodiment 2
[0069] Example 2 Preparation of GLP-1 analogue dimer with N-terminal acylation
[0070] 1) According to the monomer synthesis method in Example 1, a peptide-resin conjugate with N-terminal Fmoc-protection and side chain protection was synthesized;
[0071] 2) Remove the N-terminal Fmoc protecting group by conventional methods, suspend the resin-peptide conjugate in an appropriate amount of pyridine, add acetic anhydride or trifluoroacetic anhydride in an appropriate molar ratio, mix well, and place it to obtain the N-terminal histidine acyl Peptide-resin conjugates;
[0072] 3) Cleavage according to the method in Example 1 to obtain the crude peptide, purify, and lyophilize to obtain the monomeric target peptide;
[0073] 4) Prepare the dimer according to the method of Example 1.
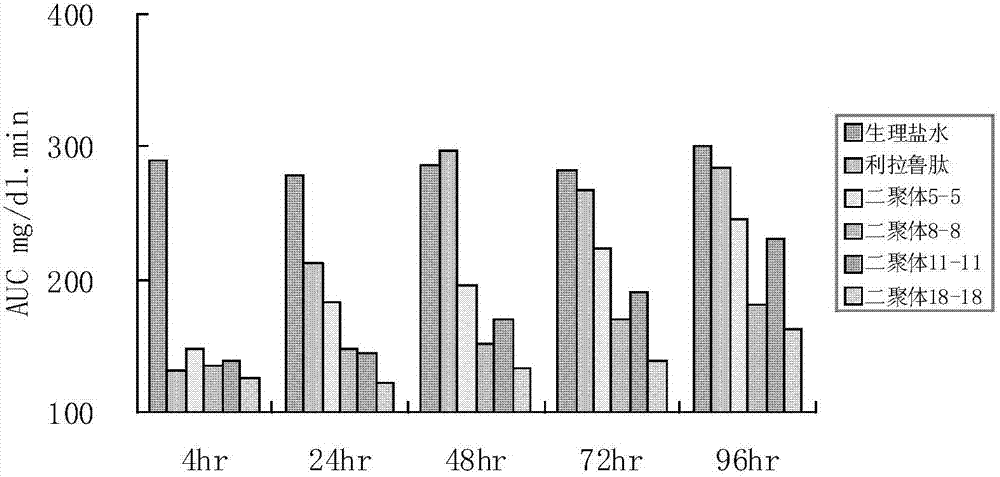
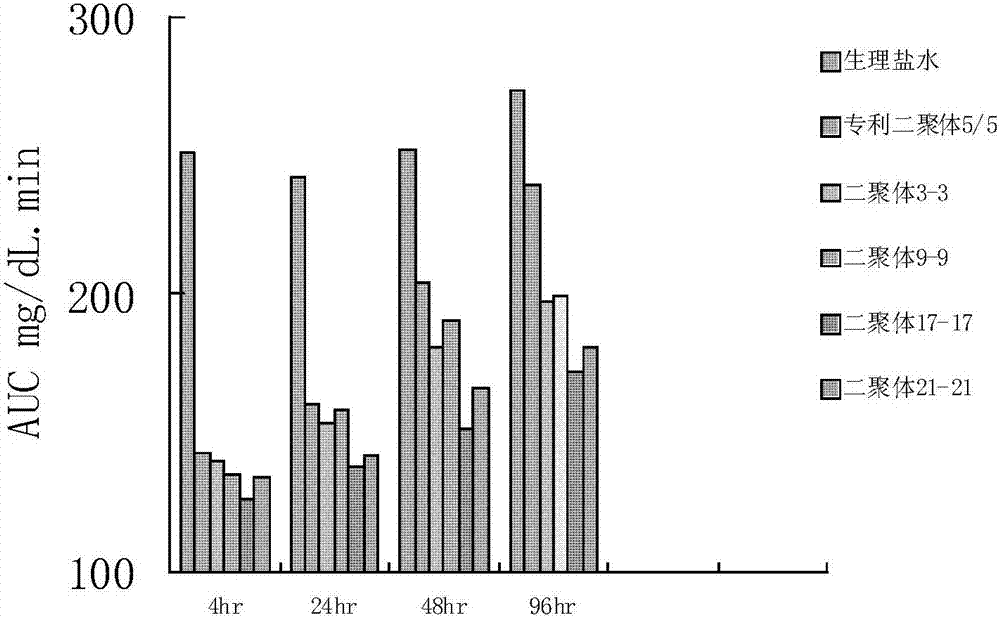
[0074] The GLP-1 analog dimer formed by the following sequence monomers was prepared according to the above method:
[0075] GLP-1 analog dimer 3-3 formed by SEQ ID NO 3 and SEQ ID NO 3,
[00...
Embodiment 3
[0082] Example 3 Preparation of GLP-1 Analog Monomers and Dimers
[0083] A. Preparation of GLP-1 analogue monomer:
[0084] 1) synthesis: adopt Fmoc method, carry out synthesis method step by step according to the following steps:
[0085] a) In the presence of an activator system, Fmoc-Gly-resin is obtained by coupling an amino resin solid-phase support and Fmoc-protected glycine;
[0086] b) Connect amino acids in accordance with the amino acid sequence of the peptide sequence by solid-phase synthesis to obtain N-terminal Fmoc-protected and side chain-protected peptide-resin conjugates; amino acids with side chains are protected as follows: tryptophan with tert-butoxy Carbonyl (Boc), glutamic acid tert-butyl (OtBu), lysine tert-butoxycarbonyl (Boc), glutamine trityl (Trt), tyrosine tert-butyl (tBu ), trityl (Trt) or tert-butyl (tBu) for serine, oxygen-tert-butyl (OtBu) for aspartic acid, tert-butyl (tBu) for threonine, trityl for histidine group (Trt) or tert-butoxycar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com