Fulvestrant or fulvestrant derivative sustained release preparation and preparation method thereof
A technology of fulvestrant and sustained-release preparations, applied in the field of pharmaceutical preparations, can solve problems such as case reports of no adverse reactions of azone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
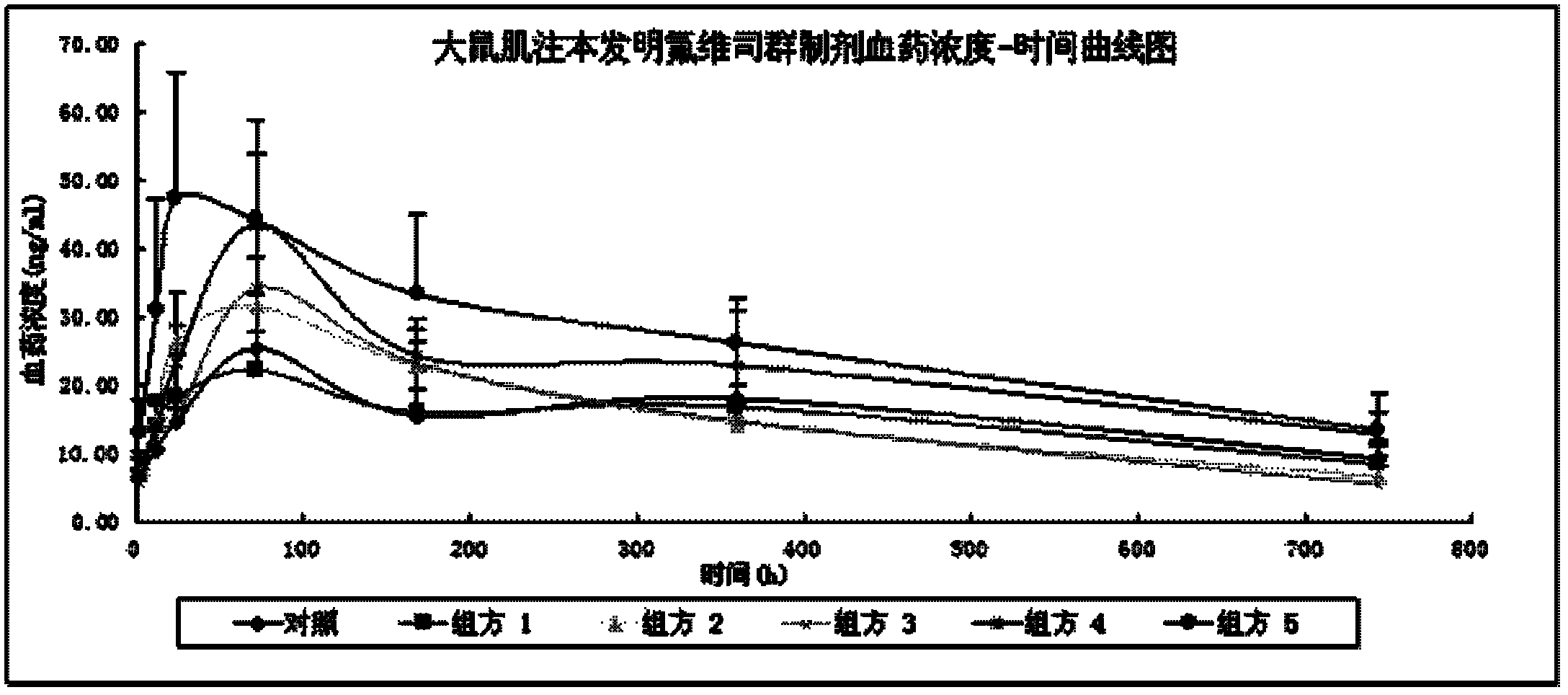
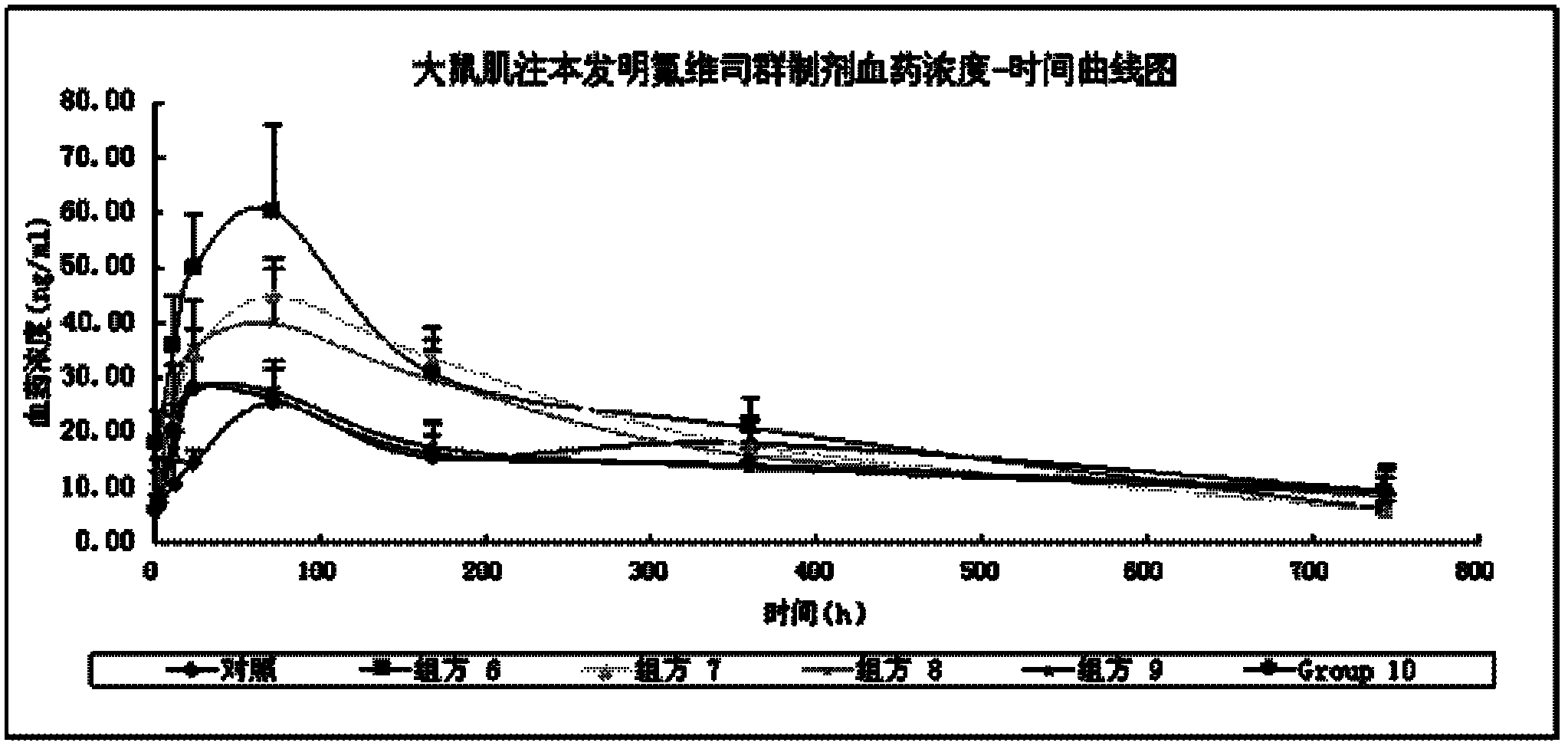
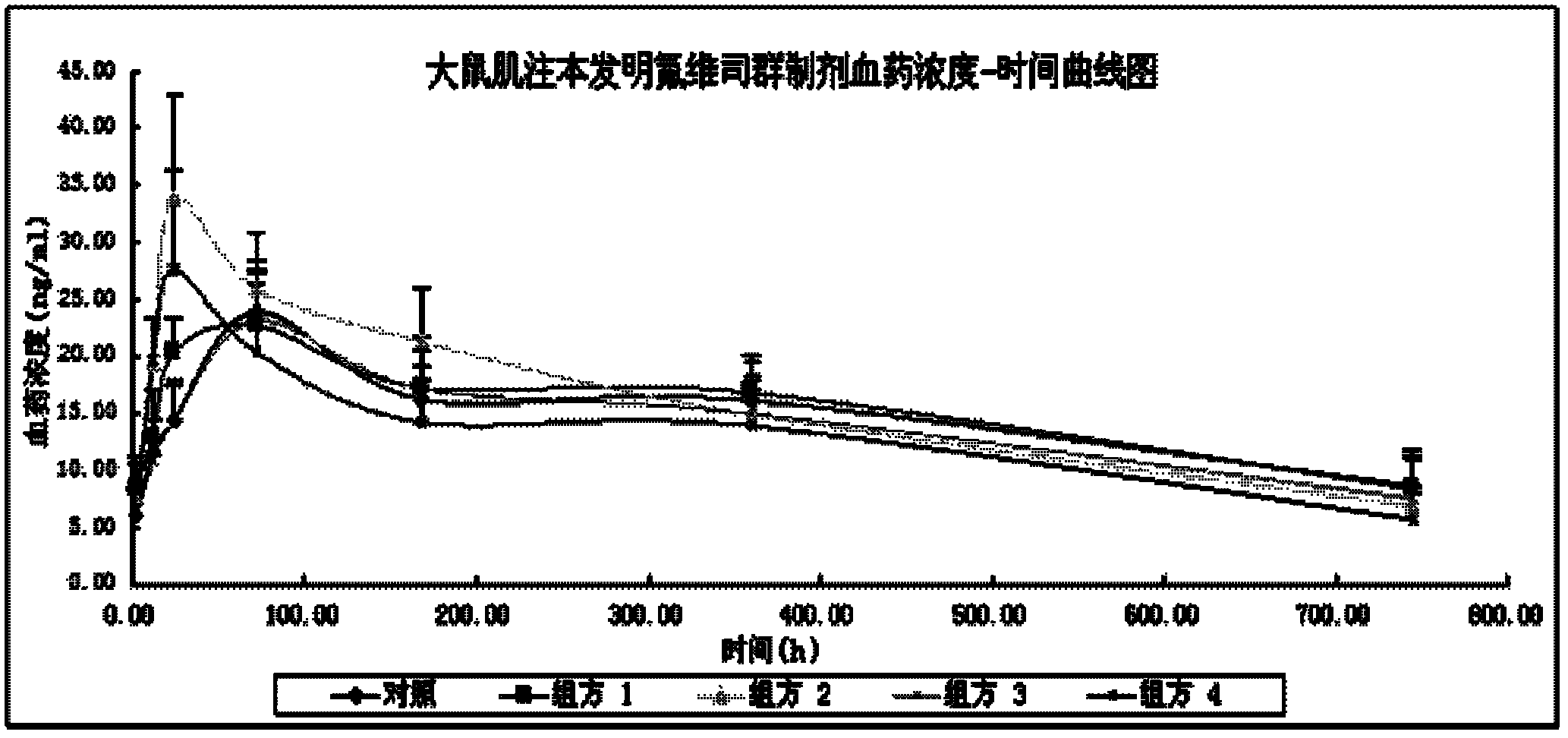
Image
Examples
experiment example 1
[0120] The solubility test of experimental example 1 fulvestrant
[0121] Experimental Instruments and Medicines
[0122] Vortex instrument, ultrasonic cleaning machine, magnetic stirrer, high performance liquid chromatography, manual pipettes of different specifications and matching nozzles, nozzle boxes, 7ml vials, matching bottle stoppers, aluminum caps.
[0123] Fulvestrant raw material, provided by the Second Pharmaceutical Factory of Xi’an Libang Pharmaceutical Co., Ltd., batch number: 080701; 1,3-butanediol, Sinopharm Chemical Reagent Co., Ltd., batch number: F20090805, imported sub-package; glycerol, Shantou City Ziguang Guhan Amino Acid Co., Ltd., batch number 060306; corn oil, North China Pharmaceutical Kangxin Co., Ltd., batch number: 081002; sunflower oil, Shanghai Jiage Food Co., Ltd., batch number: 20091113; soybean oil for injection, Xi'an Libang Pharmaceutical Co., Ltd. Provided by the First Pharmaceutical Factory; olive oil, injection grade, in bulk; rapeseed...
experiment example 2
[0129] Experimental Example 2 Miscibility test of different solvents in vitro
[0130] Divide the above-mentioned different solvents into oily solvents and non-oil-soluble solvents, accurately measure 0.5ml of each of the different solutions, mix each other, vortex for 5 minutes, and stand still for 10 minutes, observe and record the mutual miscibility of different solvents, the next day Observe and verify again, the results are as follows:
[0131] Table 2 Miscibility statistics between two different solvents
[0132]
[0133] Remarks: Take 0.5ml of each of the two solvents, mix with each other, observe the miscibility at rest, the experimental temperature is room temperature, the mixed oil is a mixture of soybean oil and castor oil, N-methylpyrrolidone is also called N-methyl-2 - pyrrolidone.
[0134] The experimental results show that oil-soluble azone and N-methyl-2-pyrrolidone can choose different oil solutions as dispersants, and dimethyl sulfoxide can only choose cas...
experiment example 3
[0135] Experimental Example 3 Miscibility test of different excipients in vitro
[0136] With reference to Experimental Example 1 and 2 experimental results, oil-soluble azone, dimethyl sulfoxide, N-methyl-2-pyrrolidone are respectively used as cosolvents, benzyl benzoate, ethyl oleate, glyceryl triacetate, Soybean oil and castor oil were used as dispersants, and benzyl alcohol and chlorobutanol were used as analgesics. The miscibility tests of different excipients were carried out, and the results are shown in Table 3.
[0137] Table 3 Miscibility and dissolution statistics among cosolvents, dispersants, and analgesics
[0138]
[0139] Remarks: Take 0.5ml of different solvents, miscible, observe the miscibility at rest, the ratio of mixed oil is 1:1, the experimental temperature is room temperature, W is benzyl alcohol, U is chlorobutanol.
[0140] The experimental results show that oil-soluble azone and N-methyl-2-pyrrolidone can choose ethyl oleate, benzyl benzoate, gl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com