Glucagon-like peptide-1 analog and uses thereof
A technology for glucagon and analogues, which is applied in the field of glucagon-like peptide-1 analogues and their medical applications, which can solve the problems of affecting drug efficacy, weakening the binding effect of peptides and receptors, and limiting drug loading of molecules To achieve the effect of reducing dosage, improving clinical compliance, and strong hypoglycemic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
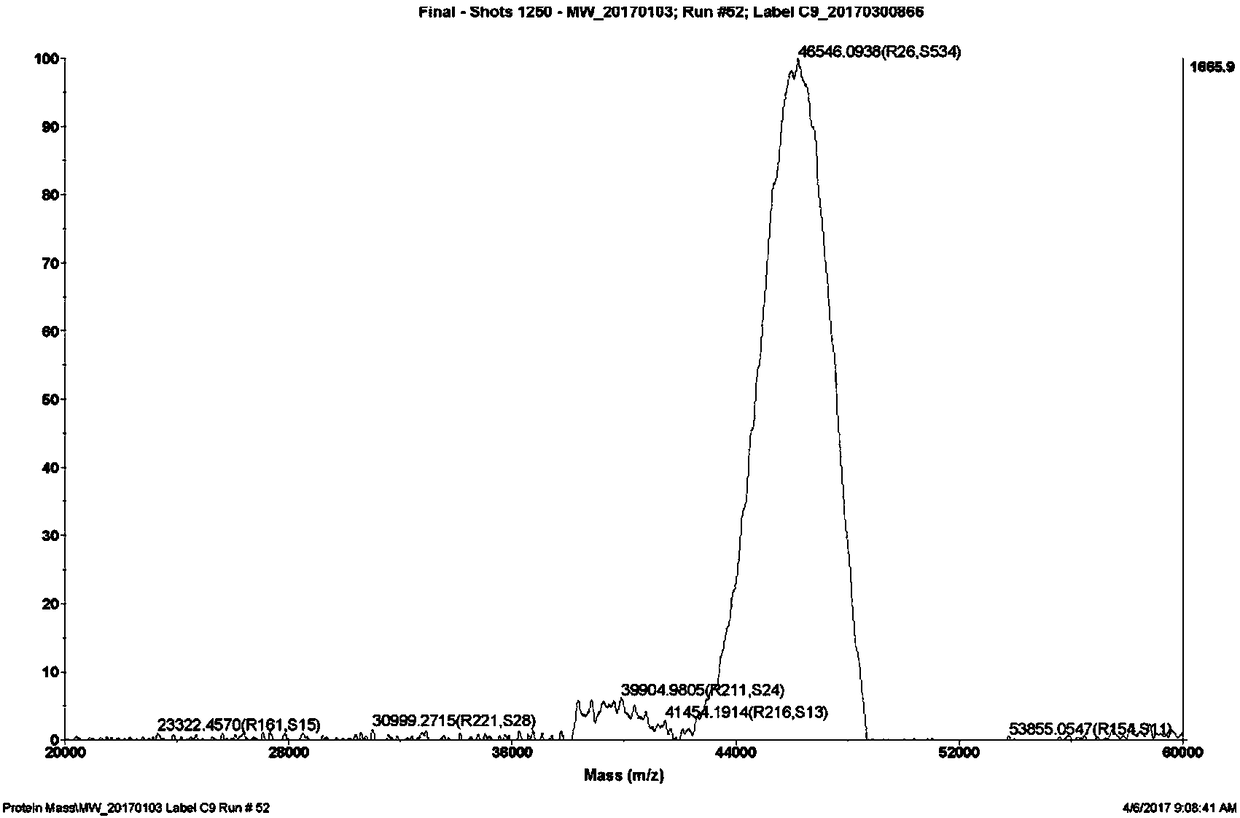
[0101] Example 1 Preparation of Glucagon-Like Peptide-1 Analogue Precursor Polypeptide
[0102] The precursor polypeptide shown in general formula I is prepared by the following steps
[0103] 1) Synthesis: Using the Fmoc strategy, use the CS 336 peptide synthesizer (CS Bio) to synthesize step by step according to the following steps:
[0104] a) In the presence of an activator system, the Fmoc-amino acid-resin is obtained by coupling the resin solid phase carrier and the Fmoc-protected C-terminal amino acid; wherein, the synthesis of the C-terminal amidated polypeptide uses an amino resin, such as Rink Amide AM, Rink Amide , Rink MBHA et al.
[0105] b) Extension of the peptide chain: Connect amino acids in accordance with the amino acid sequence of the peptide sequence by solid-phase synthesis to obtain a peptide-resin conjugate with N-terminal and side chain protection; take the following protection measures for amino acids with side chains: use Boc for tryptophan , OtB...
Embodiment 2
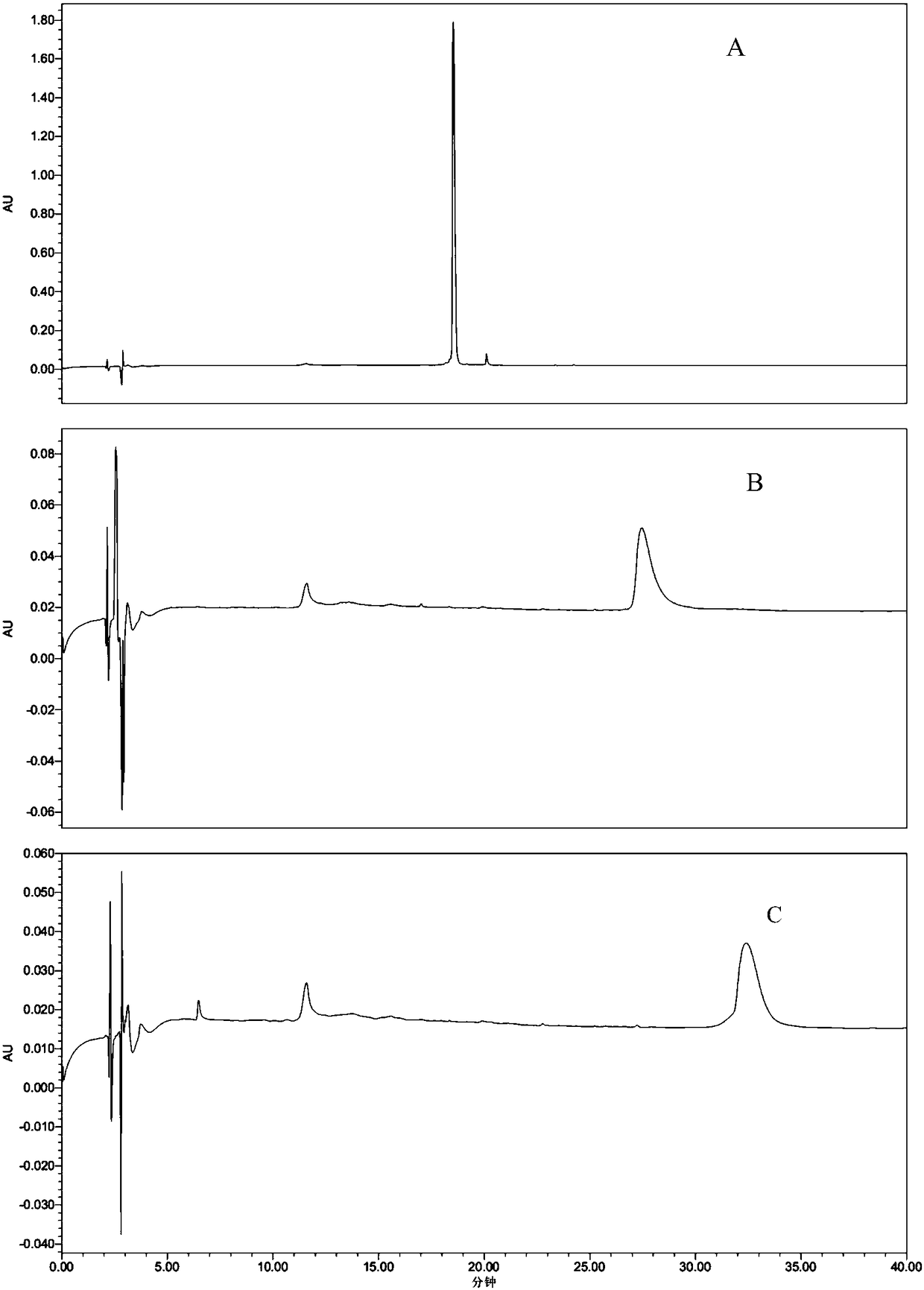
[0165] Example 2 Solubility and Stability Experiments of Glucagon-Like Peptide-1 Analogue Precursor Polypeptide
[0166] The preferred polypeptides provided by the present invention have been evaluated for solubility and solution stability, and at the same time compared with the polypeptide sequence (Gly2, Cys37GLP-1 (7-37): SEQ ID NO: 57HGEGTFTSDVSSYLEGQAAKEFIAWLVKGRC with only a simple transformation of the original sequence of GLP-1 ) and the sequence involved in the patent (CN201610211144.0) (Gly2, Aib35, Ala38, Cys39GLP-1(7-37):SEQ ID NO:58
[0167] HGEGTFTSDVSSYLEGQAAKEFIAWLVKAibRGAC) were compared and found that the structural transformation in the technical solution of the present invention effectively solved the solubility and solution stability of the precursor polypeptide.
[0168] sample:
[0169] SEQ ID NO:57, SEQ ID NO:58,
[0170] SEQ ID NO:1, 9, 11, 15, 17, 24, self-made, purity ≥ 98%.
[0171] Solubility:
[0172] Prepare the above sample solution with a...
Embodiment 3
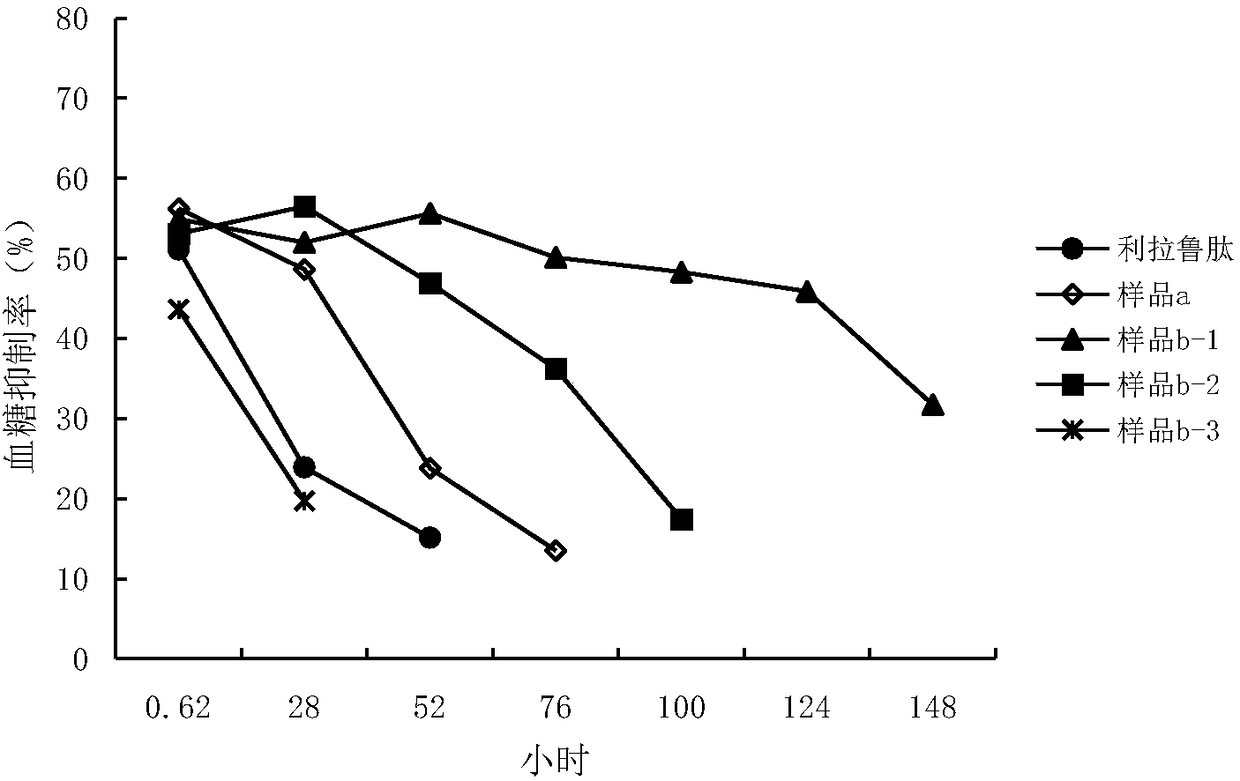
[0181] Example 3 Evaluation of the Hypoglycemic Effect of the Precursor Polypeptide of Glucagon-Like Peptide-1 Analogue
[0182] The hypoglycemic effect of some polypeptides provided by the present invention was evaluated by using a normal mouse glucose load model.
[0183] Samples tested:
[0184] SEQ ID NO:57, SEQ ID NO:58, SEQ ID NO:4, 9, 11, 15, 17, 19 self-made, purity ≥ 98%
[0185] method:
[0186] Animals (n=8) fasted overnight before the experiment, subcutaneously injected normal saline (10mL / kg) as the control group; subcutaneously injected a single liraglutide (200 μg / kg) as the positive control drug group; measured blood glucose before administration , give positive drug and test sample (200μg / kg), intraperitoneal injection of glucose (4.5g / kg) at the same time and 2 and 4 hours after the administration, take blood from the tip of the tail to measure the blood glucose value 30min after the sugar is given, and calculate relative to The blood sugar suppression r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com