Novel schiff base vanadium oxide compound as well as preparation method and application thereof
A kind of rare Buddha base and compound technology, applied in the field of new rare Buddha base vanadyl compound and its preparation, can solve the problems of inconvenient life for patients, diabetes side effects without effective treatment, etc., and achieve excellent hypoglycemic activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
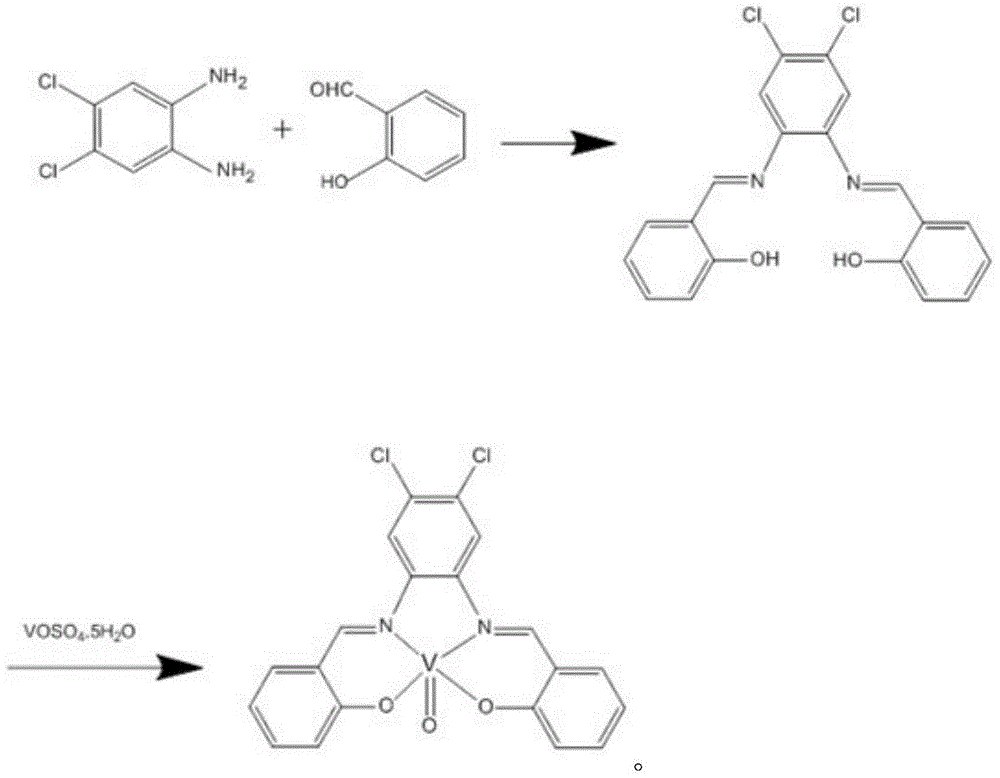
[0020] (1) Take 4,5-dichloro-o-phenylenediamine and dissolve it in ethanol solution, the molar volume ratio of 4,5-dichloro-o-phenylenediamine and ethanol is 1:30mmol / ml; take salicylaldehyde and dissolve it in ethanol solution In the method, the molar volume ratio of salicylaldehyde to ethanol is 12:15mmol / ml; drop the salicylaldehyde solution into the ethanol solution in a constant temperature water bath / oil bath at 50°C, and 4,5-dichloro-o-phenylenediamine The molar ratio with salicylaldehyde is 1:12, mixed, and the reaction is refluxed for 5 hours, a yellow flocculent precipitate is precipitated, cooled, filtered with suction, recrystallized in ethanol, the precipitate is obtained, dried, and the ligand is obtained;
[0021] (2) The ligand obtained in step (1) is dissolved in acetonitrile to obtain a ligand solution, and the molar volume ratio of the ligand to acetonitrile is 0.5:13mmol / ml; VOSO 4 ·5H 2 O is dissolved in water to obtain a metal salt solution, VOSO 4 ·5H ...
Embodiment 2
[0023] (1) Dissolve 4,5-dichloro-o-phenylenediamine in ethanol solution, the molar volume ratio of 4,5-dichloro-o-phenylenediamine to ethanol is 5:30mmol / ml; dissolve salicylaldehyde in ethanol solution In the method, the molar volume ratio of salicylaldehyde to ethanol is 6:15mmol / ml; drop the salicylaldehyde solution into the ethanol solution in a constant temperature water bath / oil bath at 70°C, and 4,5-dichloro-o-phenylenediamine The molar ratio with salicylaldehyde is 5:6, mixed, and the reaction is refluxed for 3 hours, a yellow flocculent precipitate is precipitated, cooled, suction filtered, recrystallized in ethanol to obtain a precipitate, dried, and the ligand is obtained;
[0024] (2) The ligand obtained in step (1) is dissolved in acetonitrile to obtain a ligand solution, and the molar volume ratio of the ligand to acetonitrile is 2:13mmol / ml; VOSO 4 ·5H 2 O is dissolved in water to obtain a metal salt solution, VOSO 4 ·5H 2 The molar volume ratio of O to water...
Embodiment 3
[0026] (1) Dissolve 4,5-dichloro-o-phenylenediamine (3mmol, 0.51g) in 30mL of ethanol solution, and dissolve salicylaldehyde (9mmol, 1.05g) in 15mL of ethanol solution. Add the salicylaldehyde solution drop by drop in the bath and mix the two. The reaction was refluxed for 4 hours, and yellow flocculent precipitates were precipitated, cooled and filtered with suction. Recrystallization was carried out in ethanol to obtain a precipitate, which was dried to obtain a ligand.
[0027] (2) The ligand (1mmol, 0.383g) obtained above was dissolved in 13mL MeCN, VOSO 4 ·5H 2 O (1 mmol, 0.254 g) was dissolved in 20 mL of water. Under stirring, add the metal salt solution dropwise into the ligand solution, stir for 30 minutes, a brown precipitate is produced, filter, dissolve the precipitate in DMSO, and keep the filtrate at the same time. After 30 days, the DMSO solution precipitates yellow massive complex crystals, suitable for X- X-ray single crystal diffraction. The yield was 80...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com