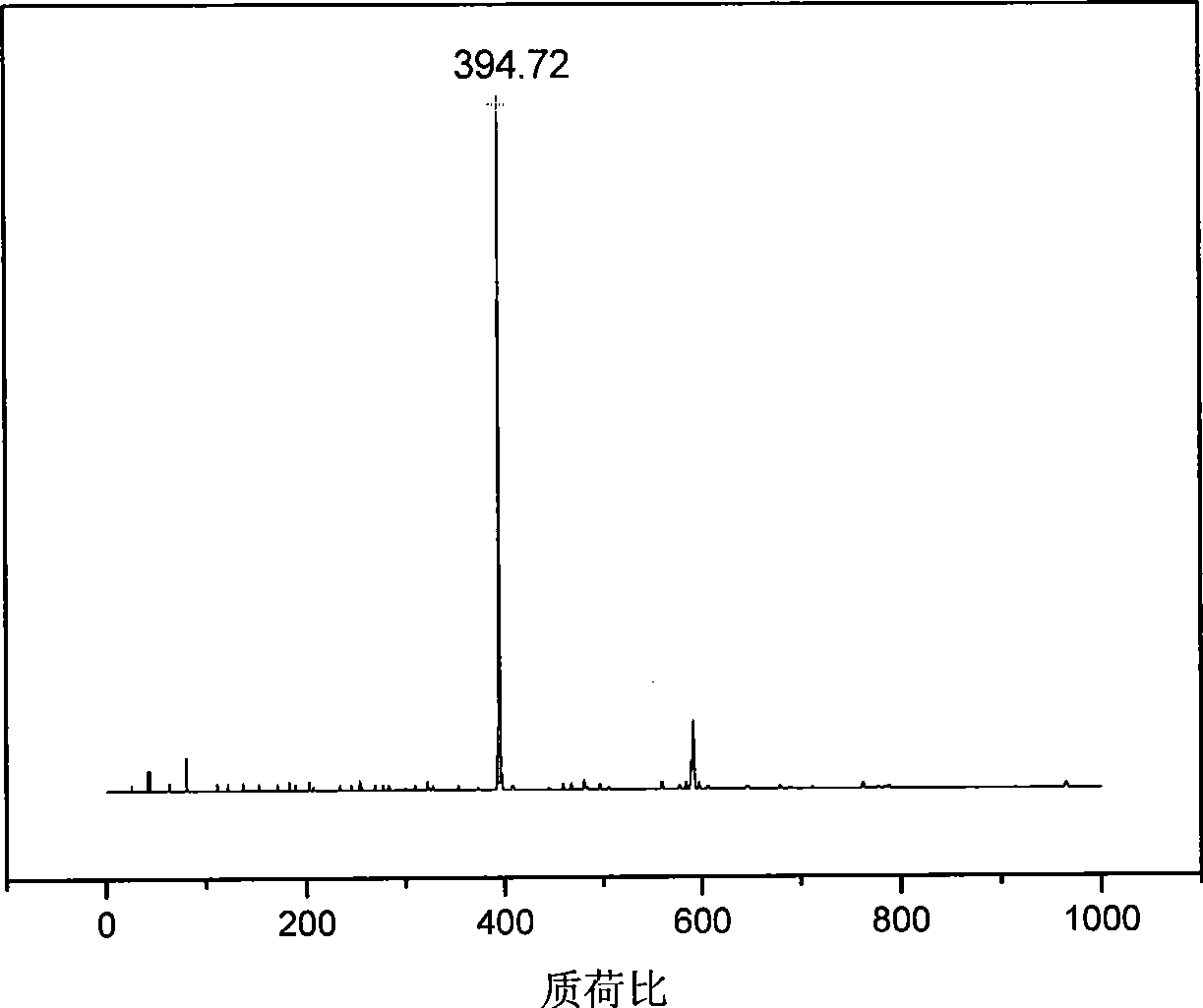
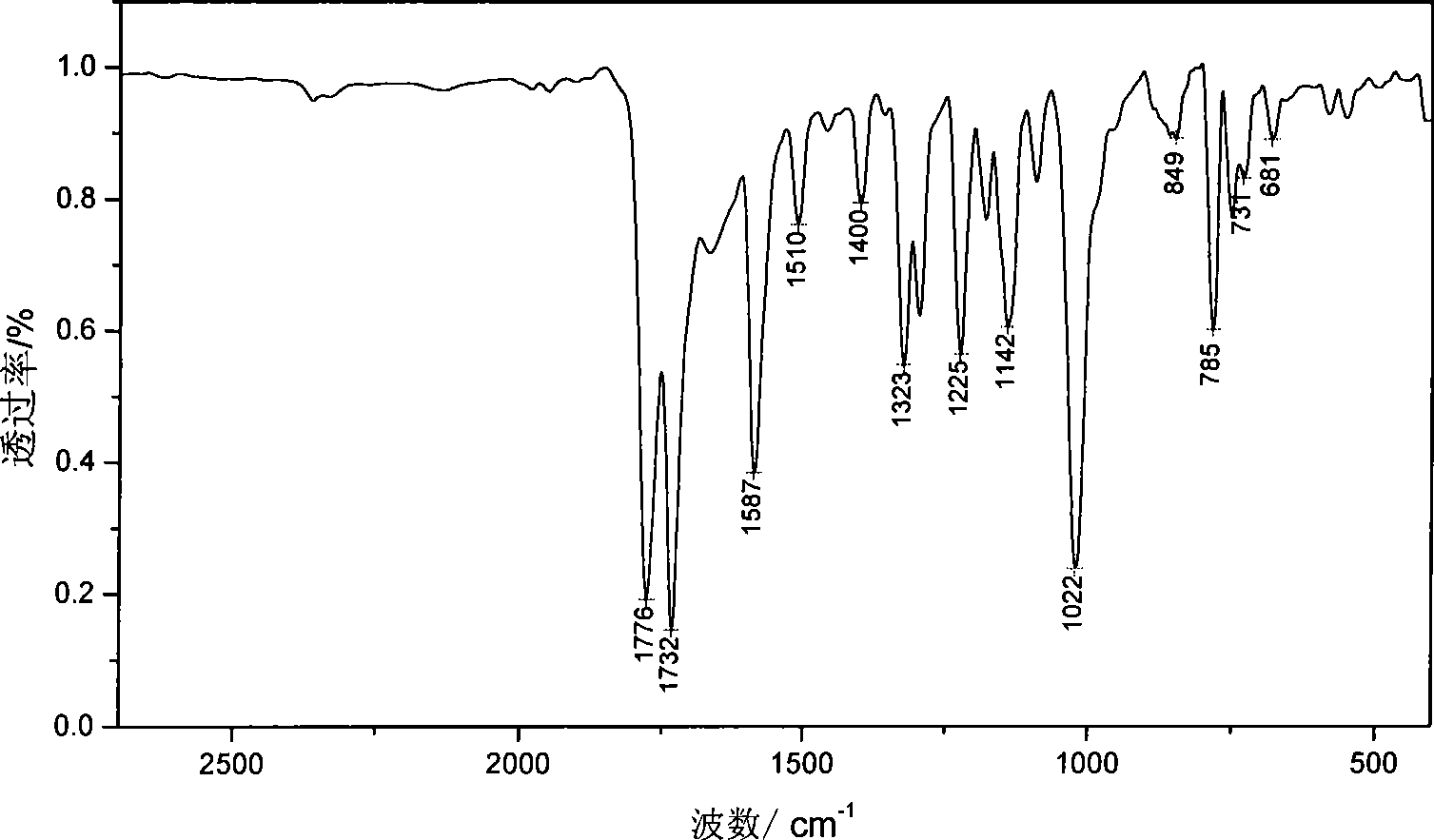
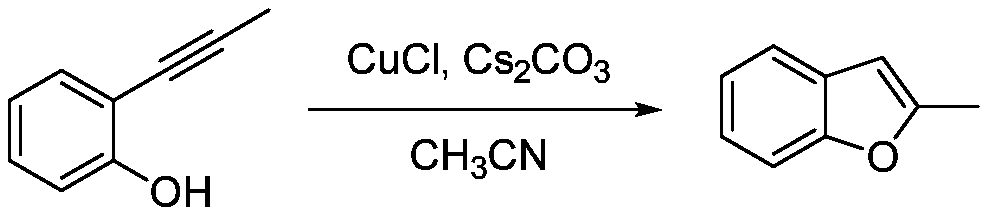Patents
Literature
73 results about "Molar volume" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The molar volume, symbol Vₘ, is the volume occupied by one mole of a substance (chemical element or chemical compound) at a given temperature and pressure. It is equal to the molar mass (M) divided by the mass density (ρ). It has the SI unit cubic metres per mole (m³/mol), although it is more practical to use the units cubic decimetres per mole (dm³/mol) for gases and cubic centimetres per mole (cm³/mol) for liquids and solids.
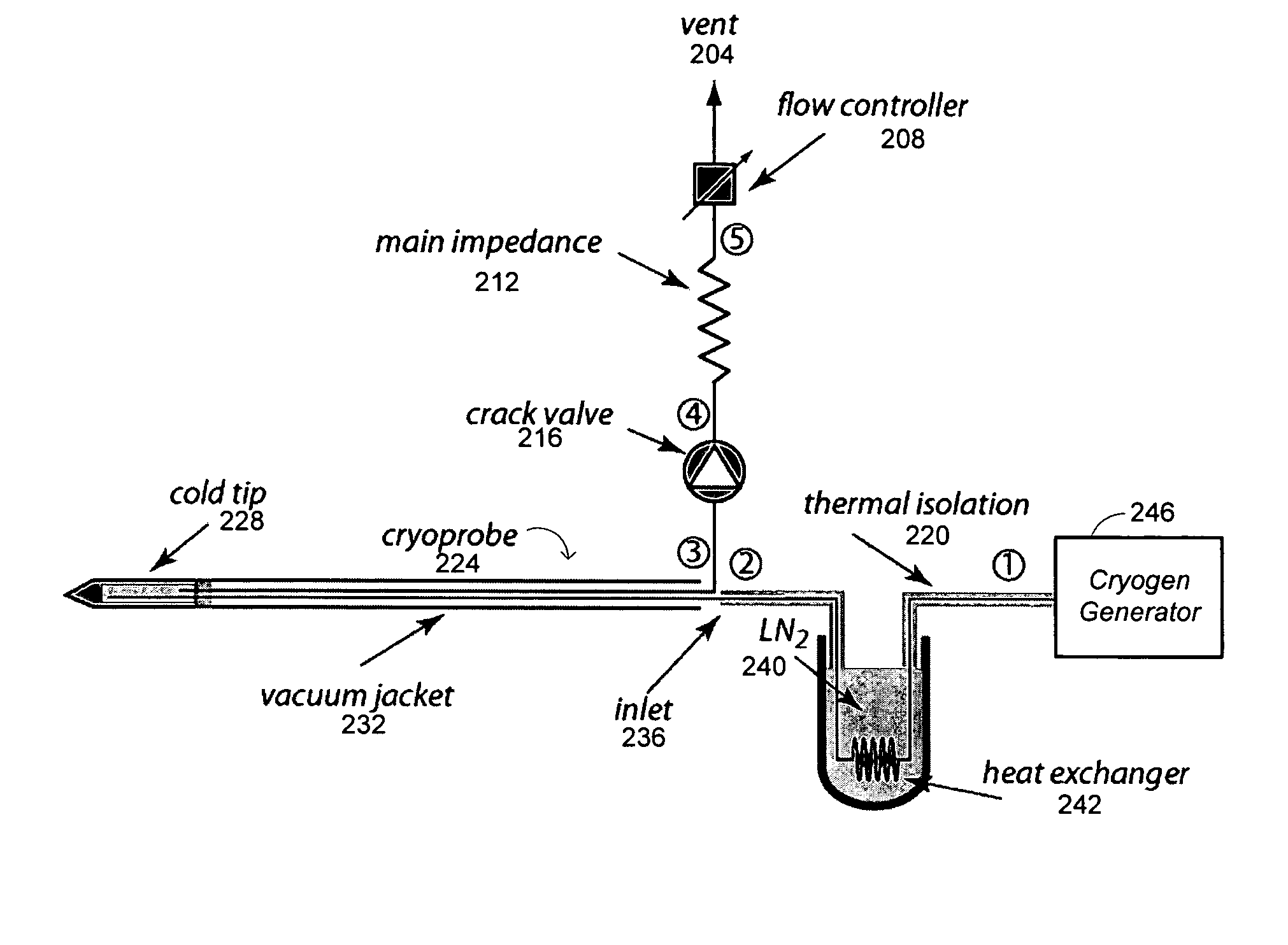
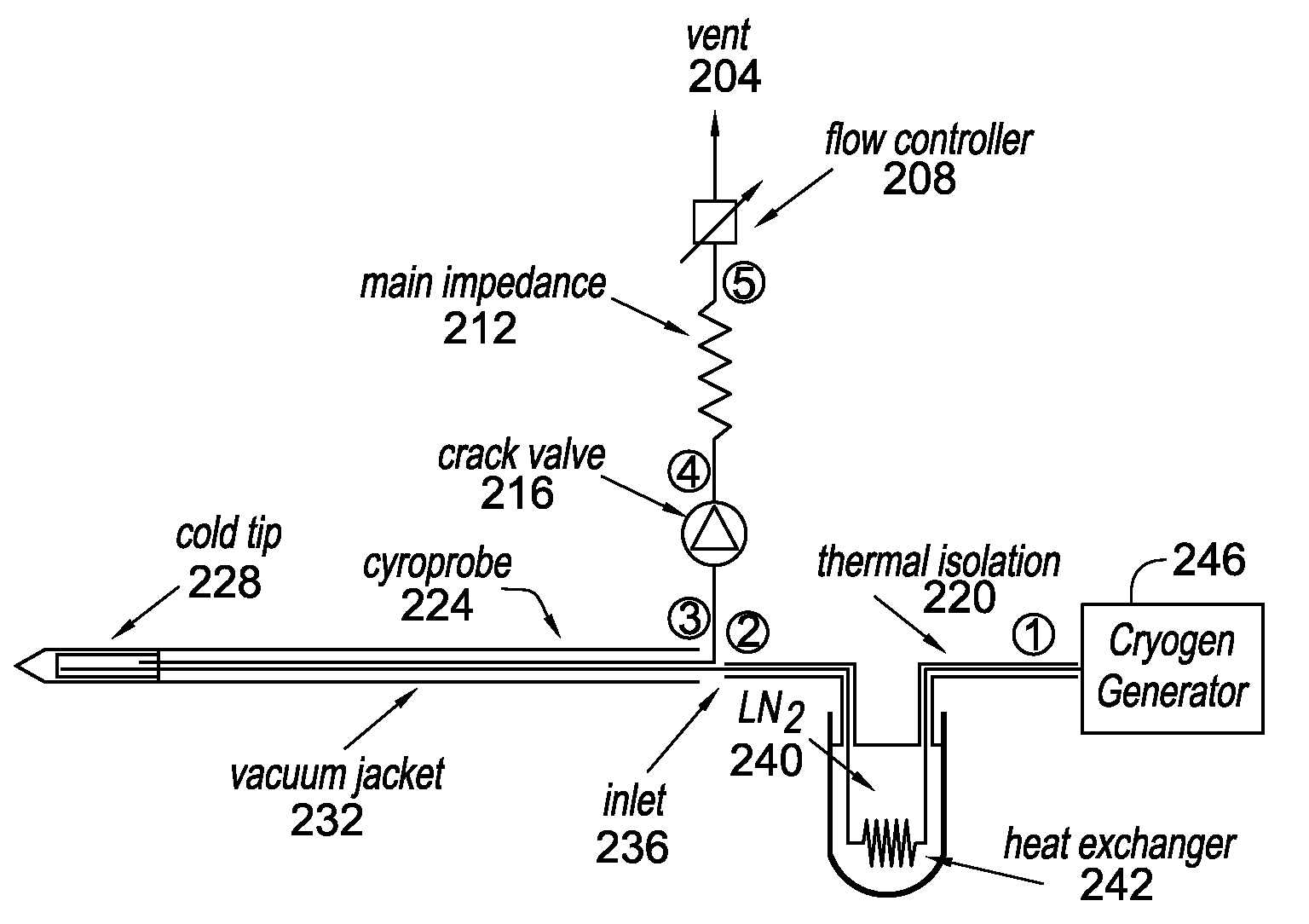
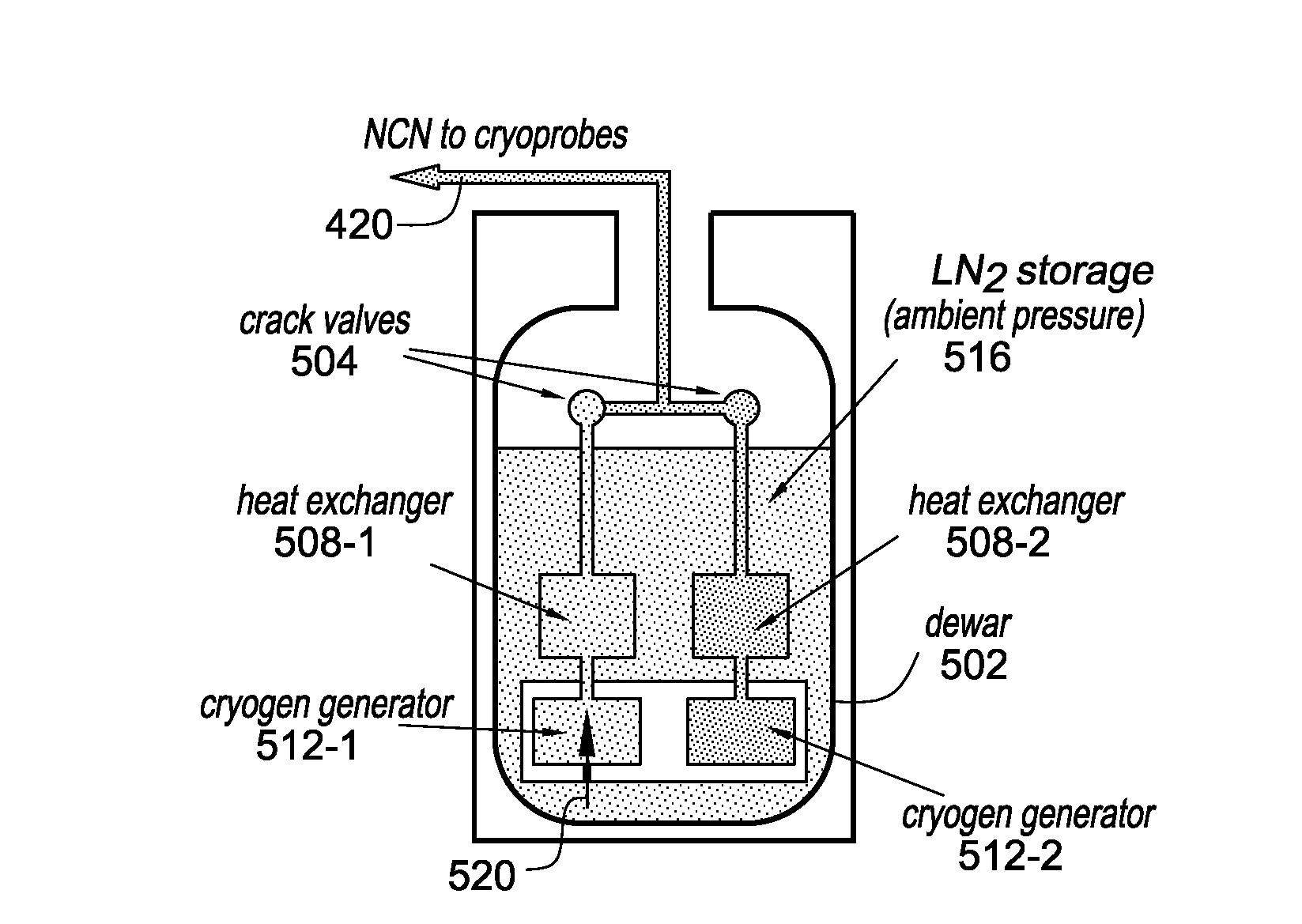
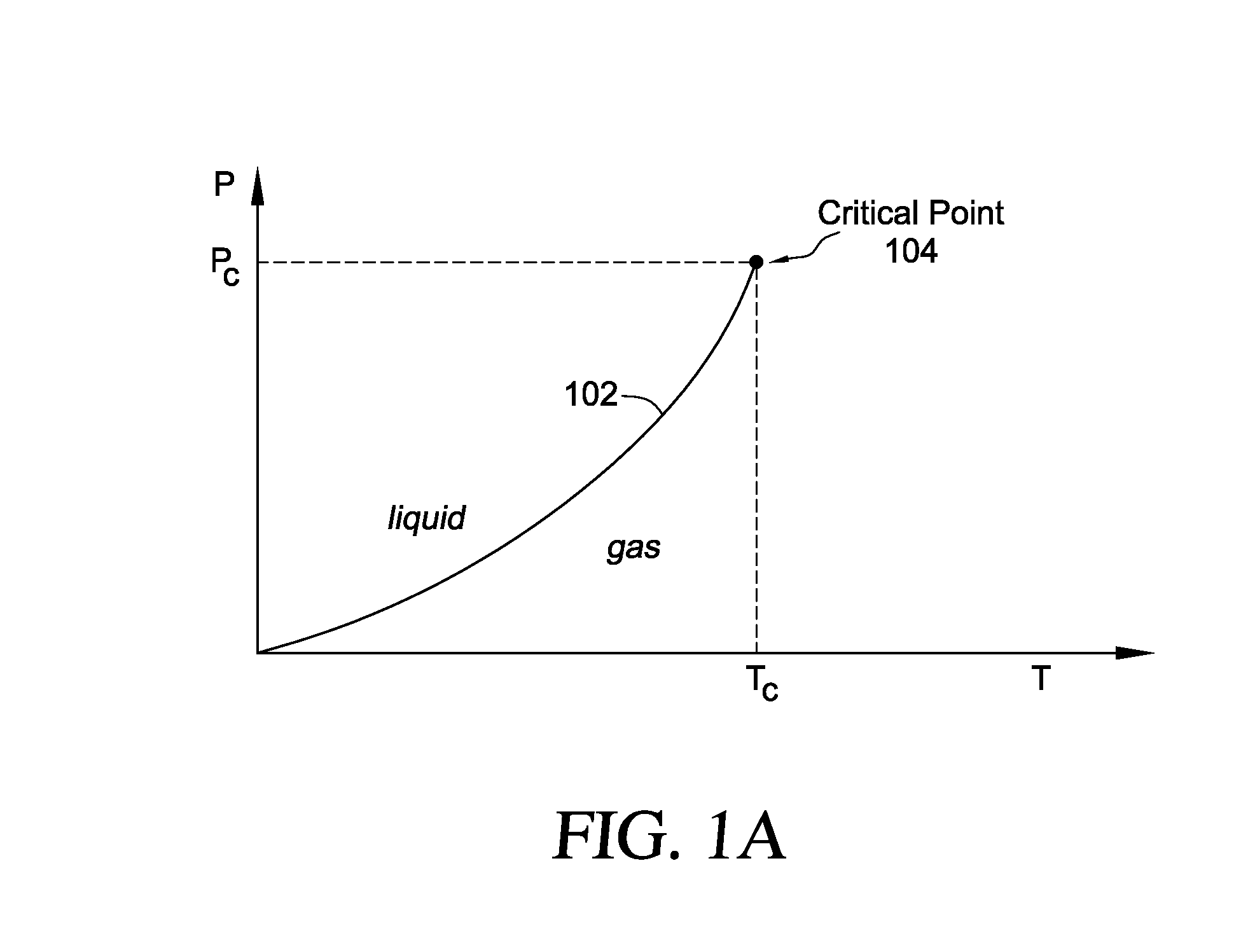
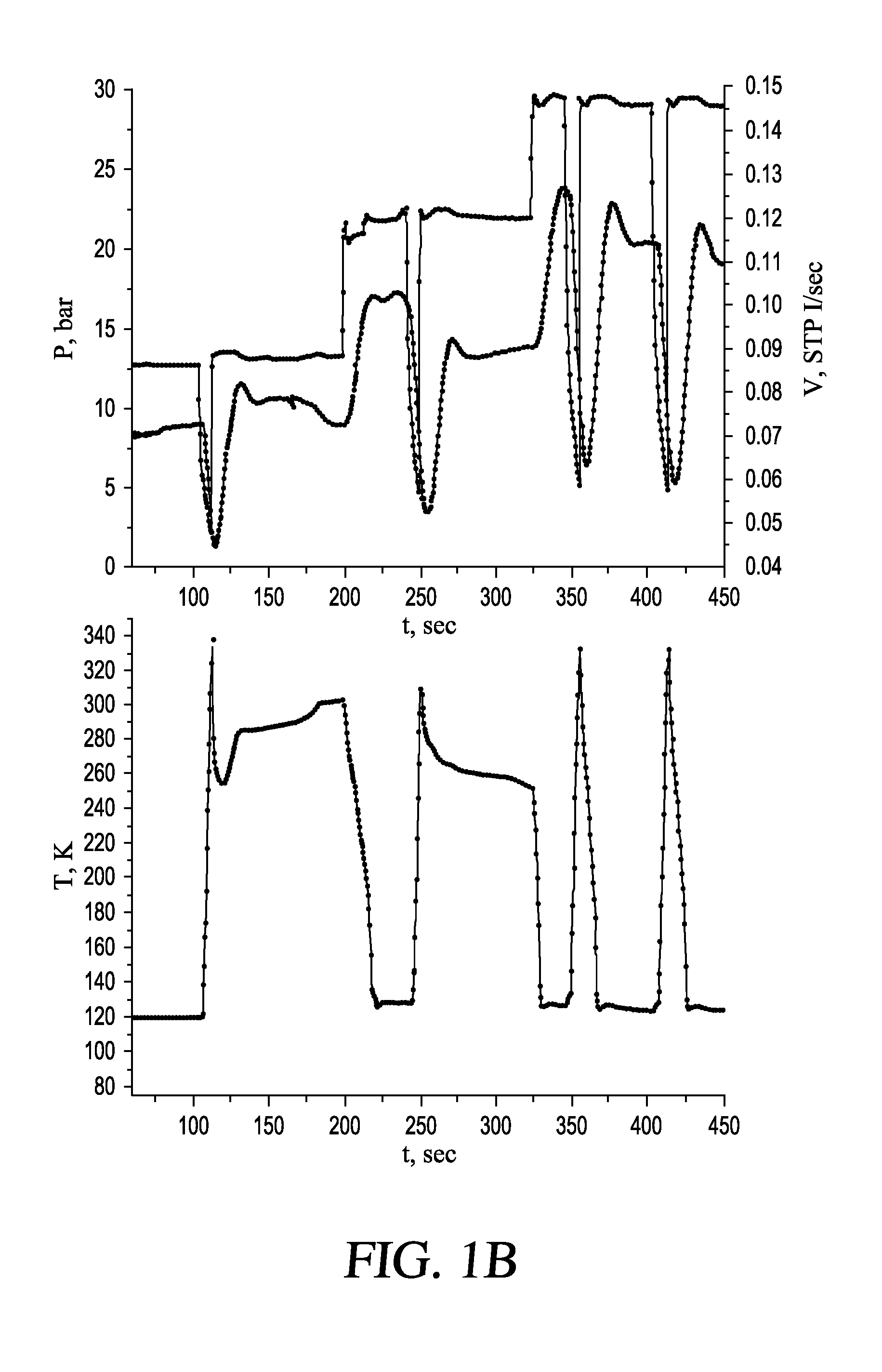
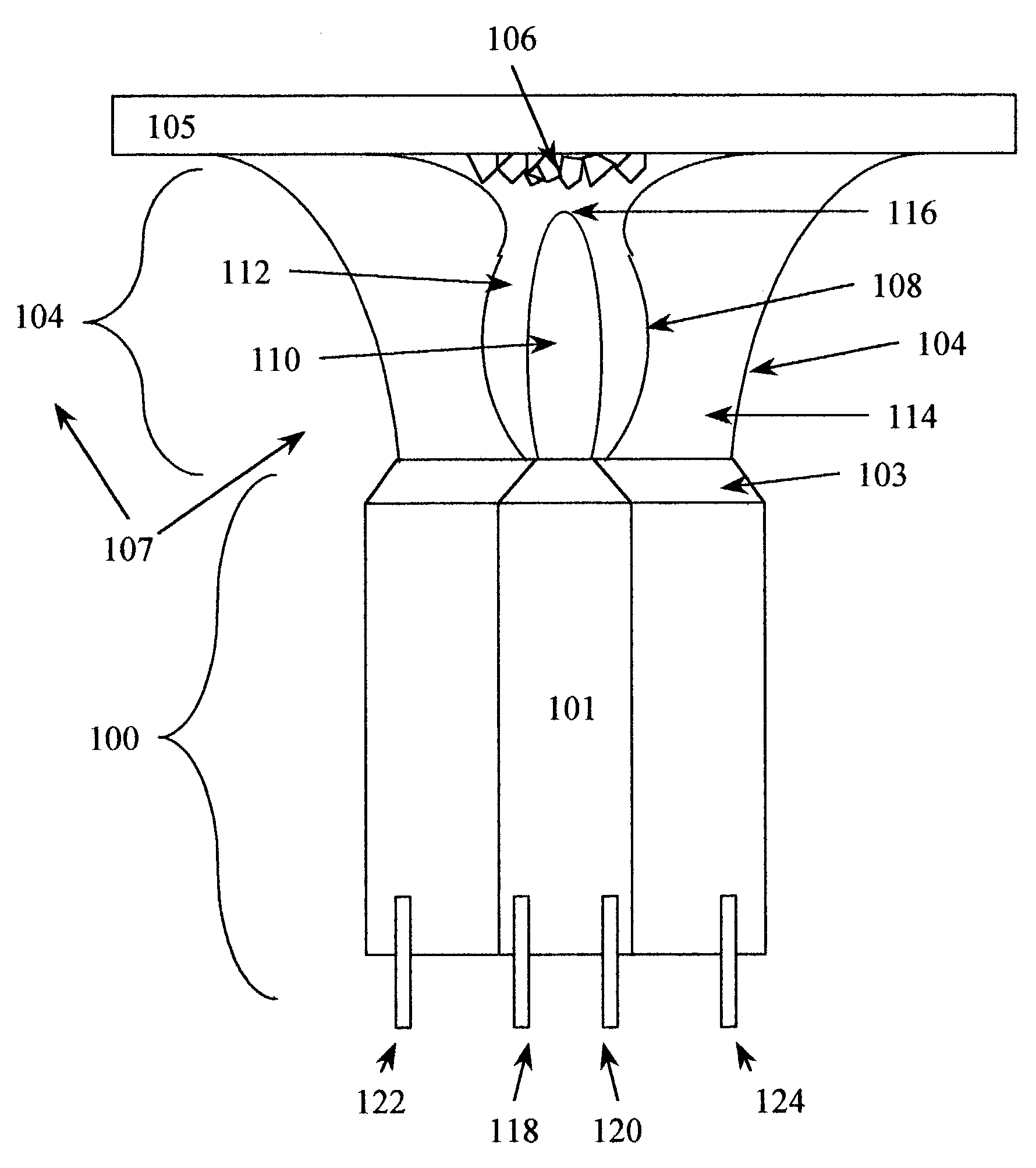
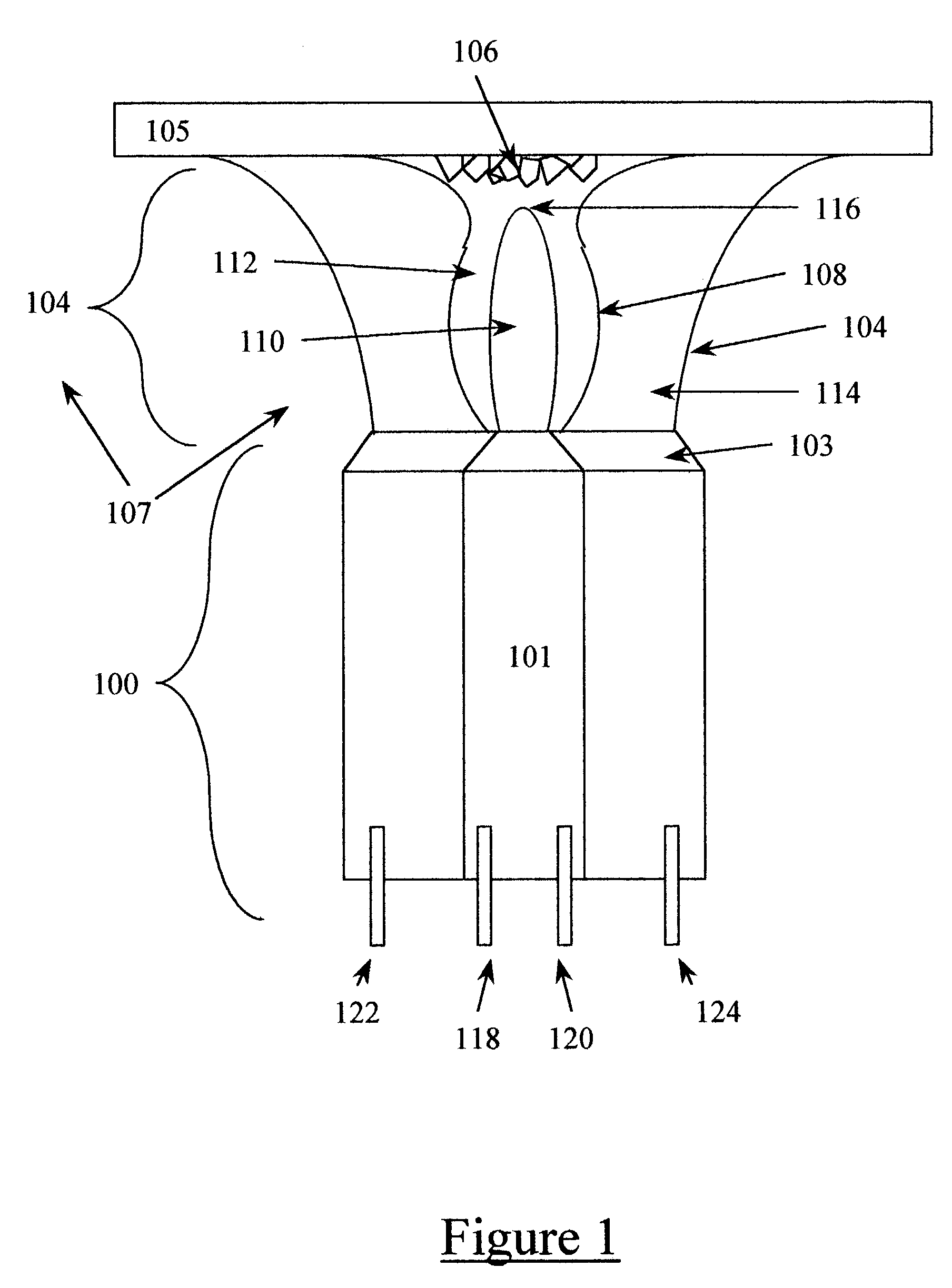
Methods and systems for cryogenic cooling
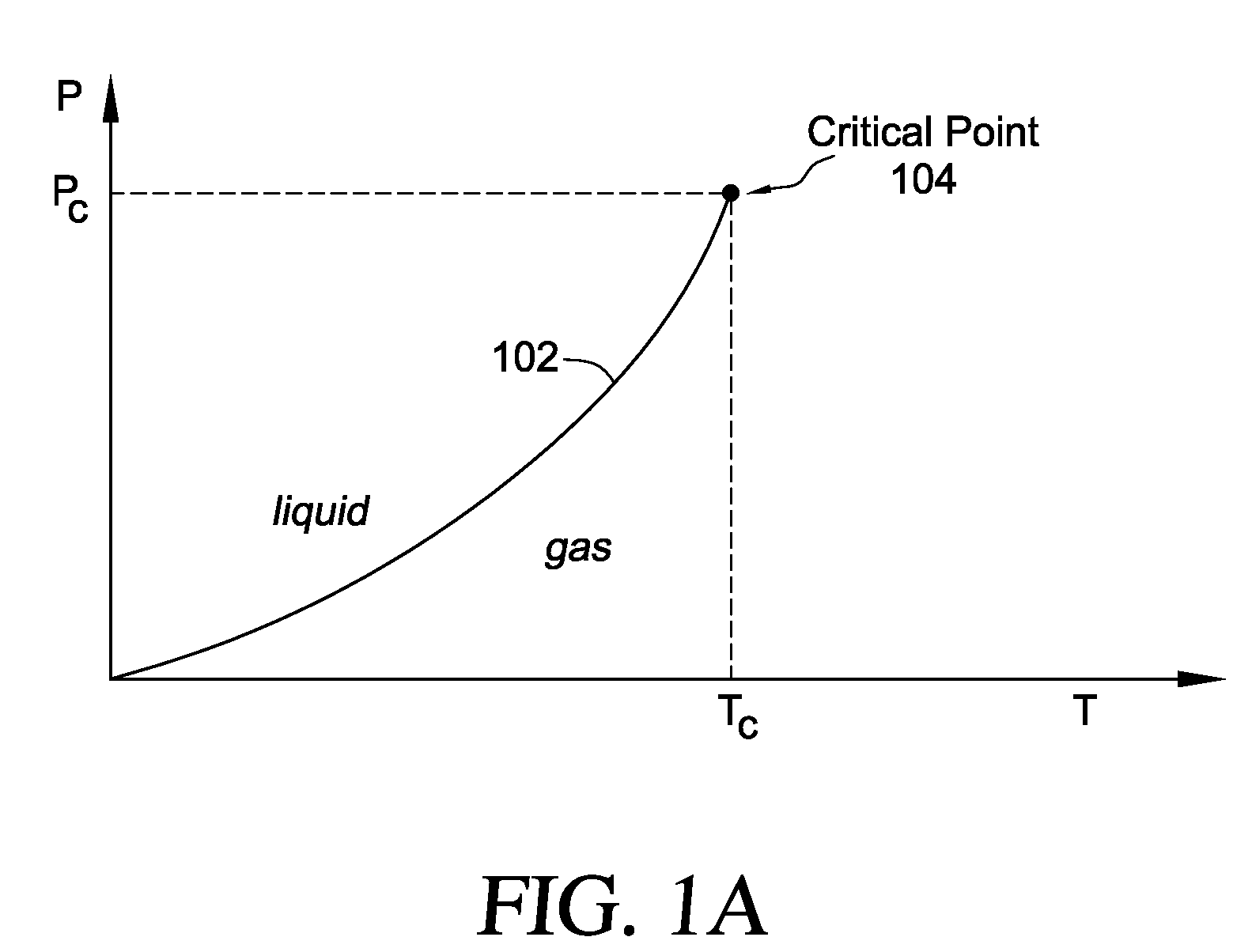
ActiveUS20050261753A1Increase pressurePrevents vapor lockDiagnosticsCompression machinesEngineeringVapor lock
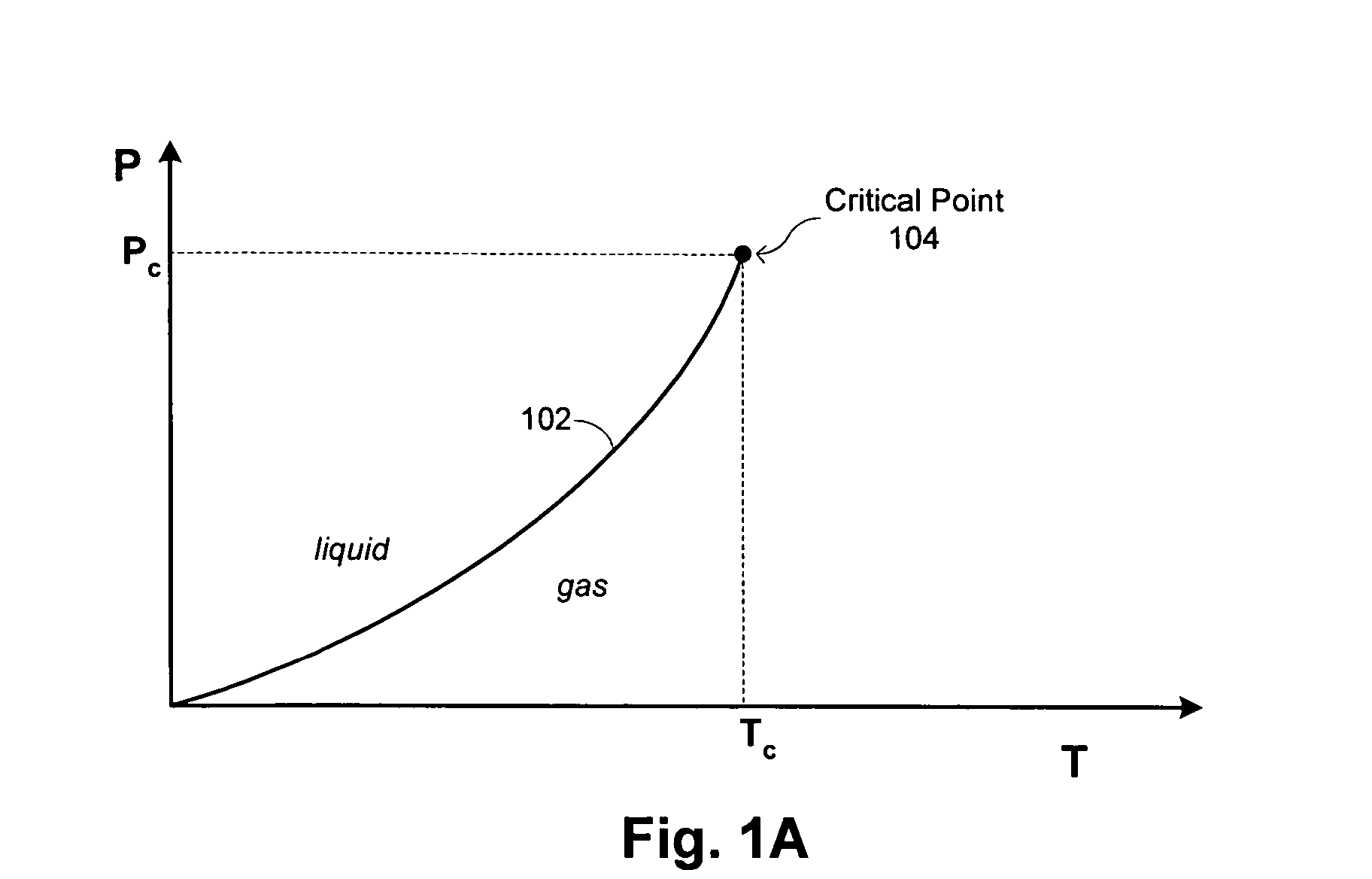
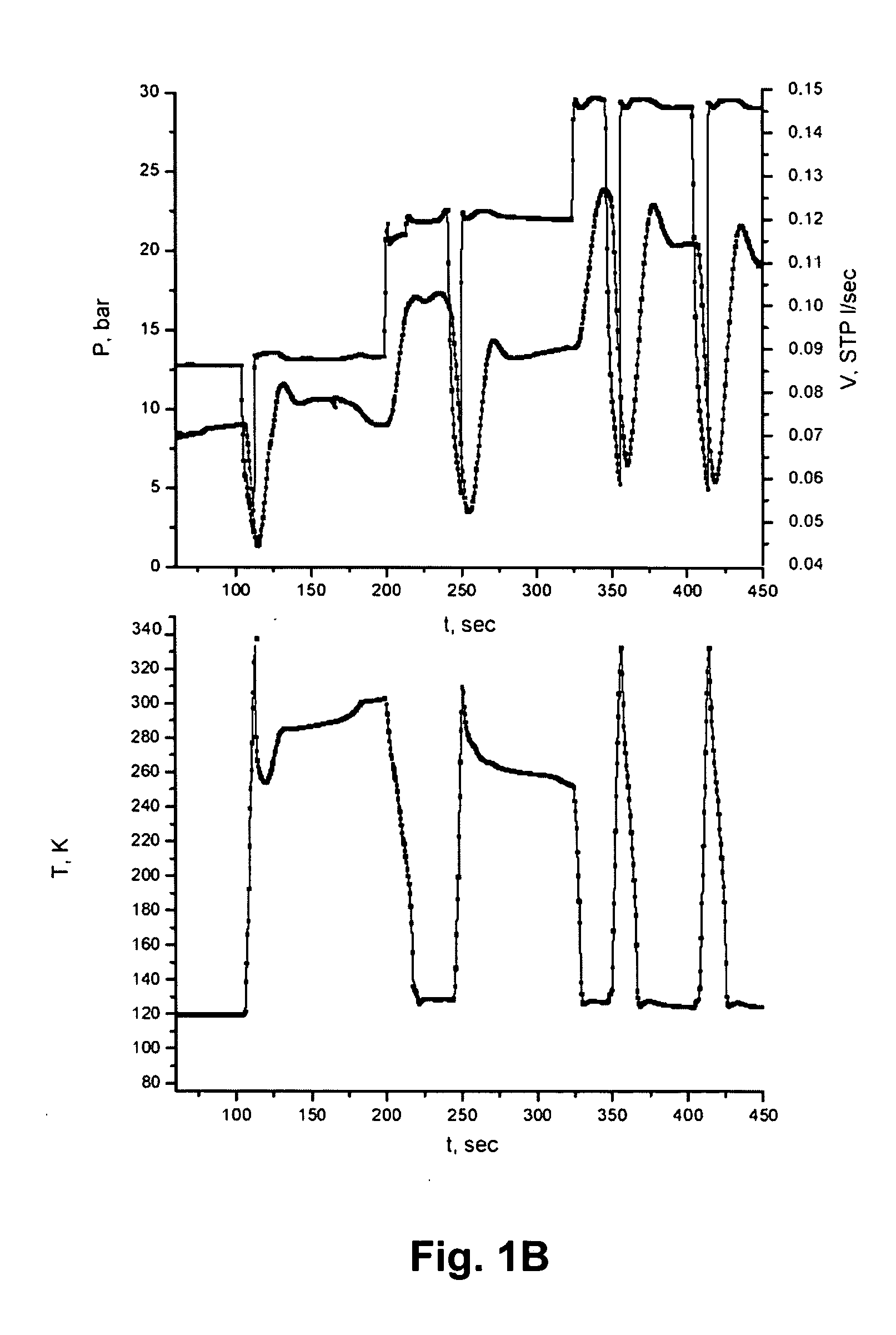
Methods and systems are provided for cooling an object with a cryogen having a critical point defined by a critical-point pressure and a critical-point temperature. A pressure of the cryogen is raised above a pressure value determined to provide the cryogen at a reduced molar volume that prevents vapor lock. Thereafter, the cryogen is placed in thermal communication with the object to increase a temperature of the cryogen along a thermodynamic path that maintains the pressure greater than the critical-point pressure for a duration that the cryogen and object are in thermal communication.
Owner:ADAGIO MEDICAL
Methods and systems for cryogenic cooling
InactiveUS7921657B2Reduce the temperatureCompression machines with non-reversible cycleTherapyEngineeringVapor lock
Methods and systems are provided for cooling an object with a cryogen having a critical point defined by a critical-point pressure and a critical-point temperature. A pressure of the cryogen is raised above a pressure value determined to provide the cryogen at a reduced molar volume that prevents vapor lock. Thereafter, the cryogen is placed in thermal communication with the object to increase a temperature of the cryogen along a thermodynamic path that maintains the pressure greater than the critical-point pressure for a duration that the cryogen and object are in thermal communication.
Owner:ADAGIO MEDICAL
Positive Electrode Active Material for Lithium Ion Battery, Positive Electrode for Secondary Battery, and Lithium Ion Battery
ActiveUS20110031437A1High crystallinityEnsure characteristicPositive electrodesLi-accumulatorsHorizontal axisManganese
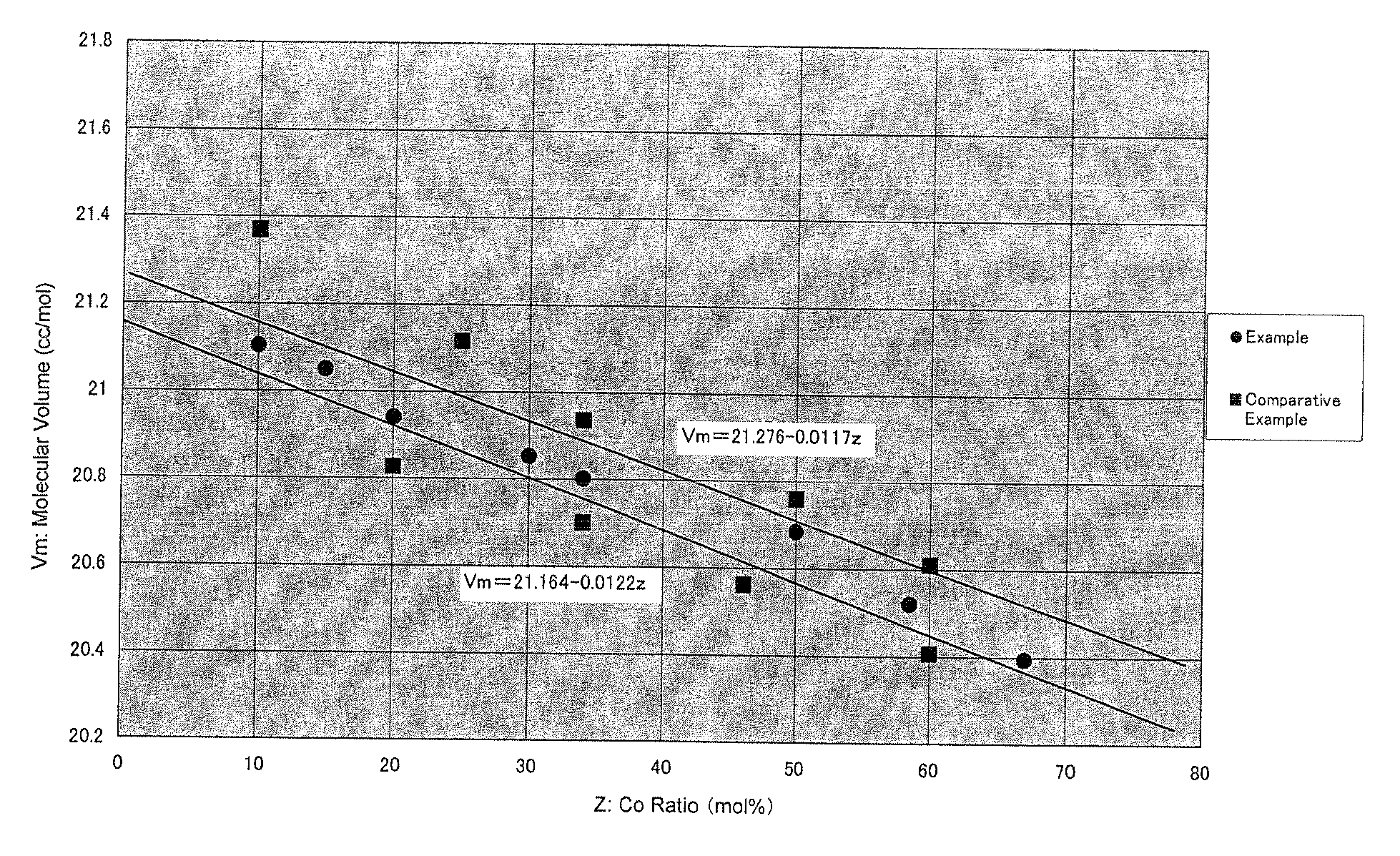
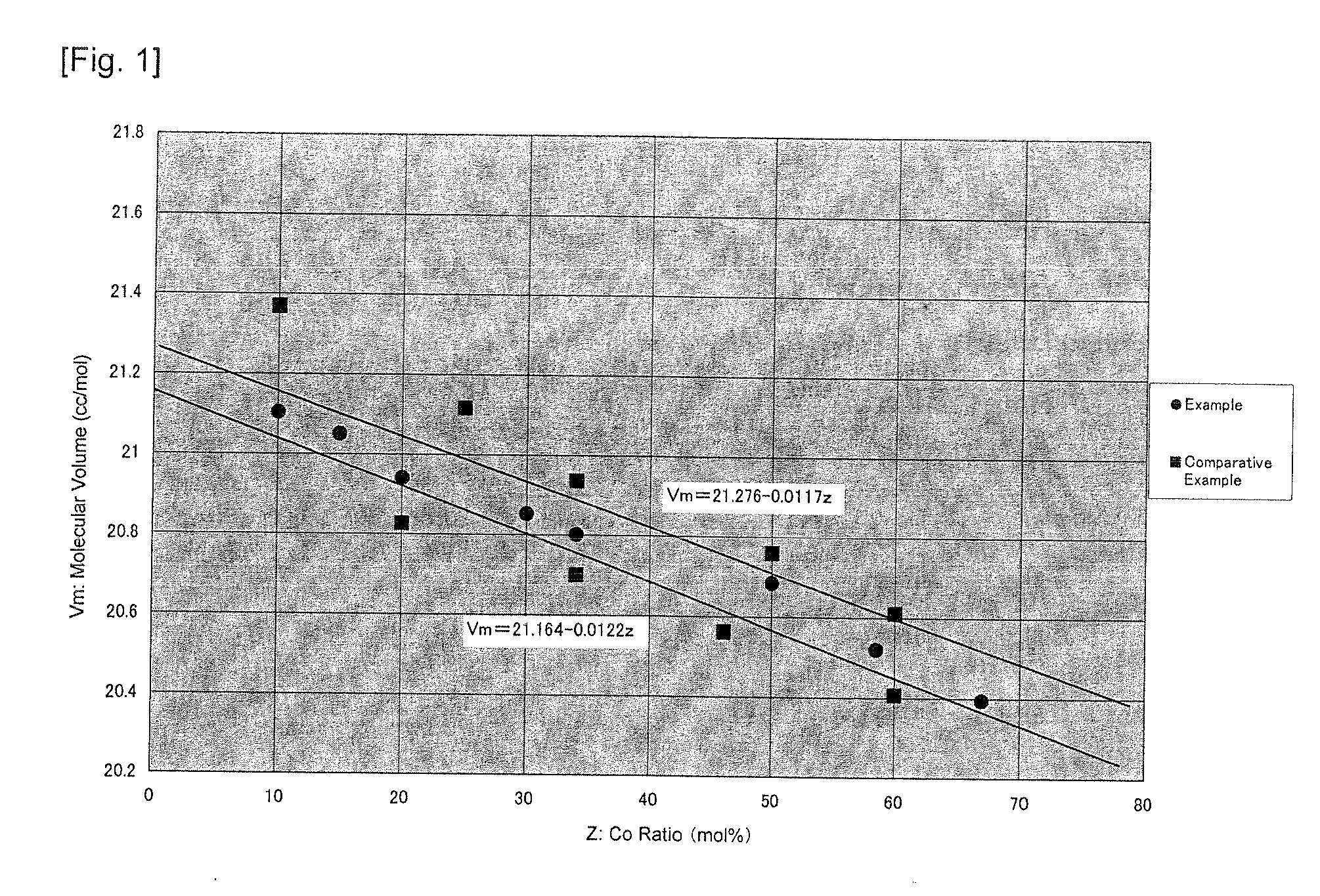
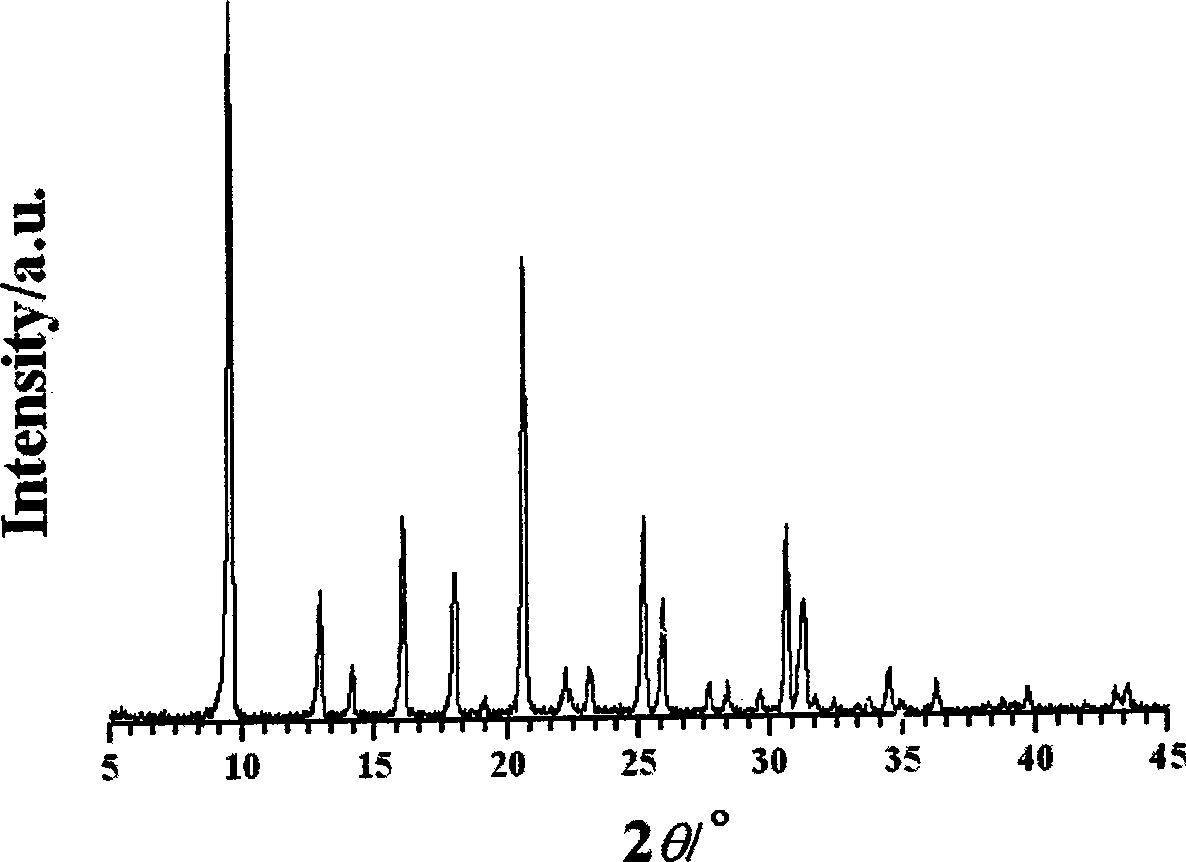
Provided is a positive electrode active material for a lithium ion battery positive electrode material made of lithium-containing nickel-manganese-cobalt composite oxide of a layered structure represented with LiaNixMnyCozO2 (1.0<a<1.3, 0.8<x+y+z<1.1), wherein, in a region with a molar volume Vm that is estimated from a lattice constant calculated from a (018) plane and a (113) plane in a powder X-ray diffraction pattern using CuK alpha rays as a vertical axis and Co ratio z (molar %) in metal components as a horizontal axis, the relationship thereof is within a range of Vm=21.276-0.0117z as an upper limit and Vm=21.164-0.0122z as a lower limit, and a half value width of both the (018) plane and the (113) plane is 0.200° or less. As a result of studying and defining the relationship of the cobalt (Co) ratio and the lattice constant, which are considered to influence the crystal structure, obtained was a positive electrode active material having high crystallinity, high capacity and high security. By using this material, it is possible to obtain a positive electrode active material for a lithium ion battery that is able to further ensure the characteristics and safety of the lithium ion battery.
Owner:JX NIPPON MINING& METALS CORP
Methods and systems for cryogenic cooling
ActiveUS20080173028A1Reduce the temperatureCompression machines with non-reversible cycleTherapyEngineeringVapor lock
Owner:ADAGIO MEDICAL
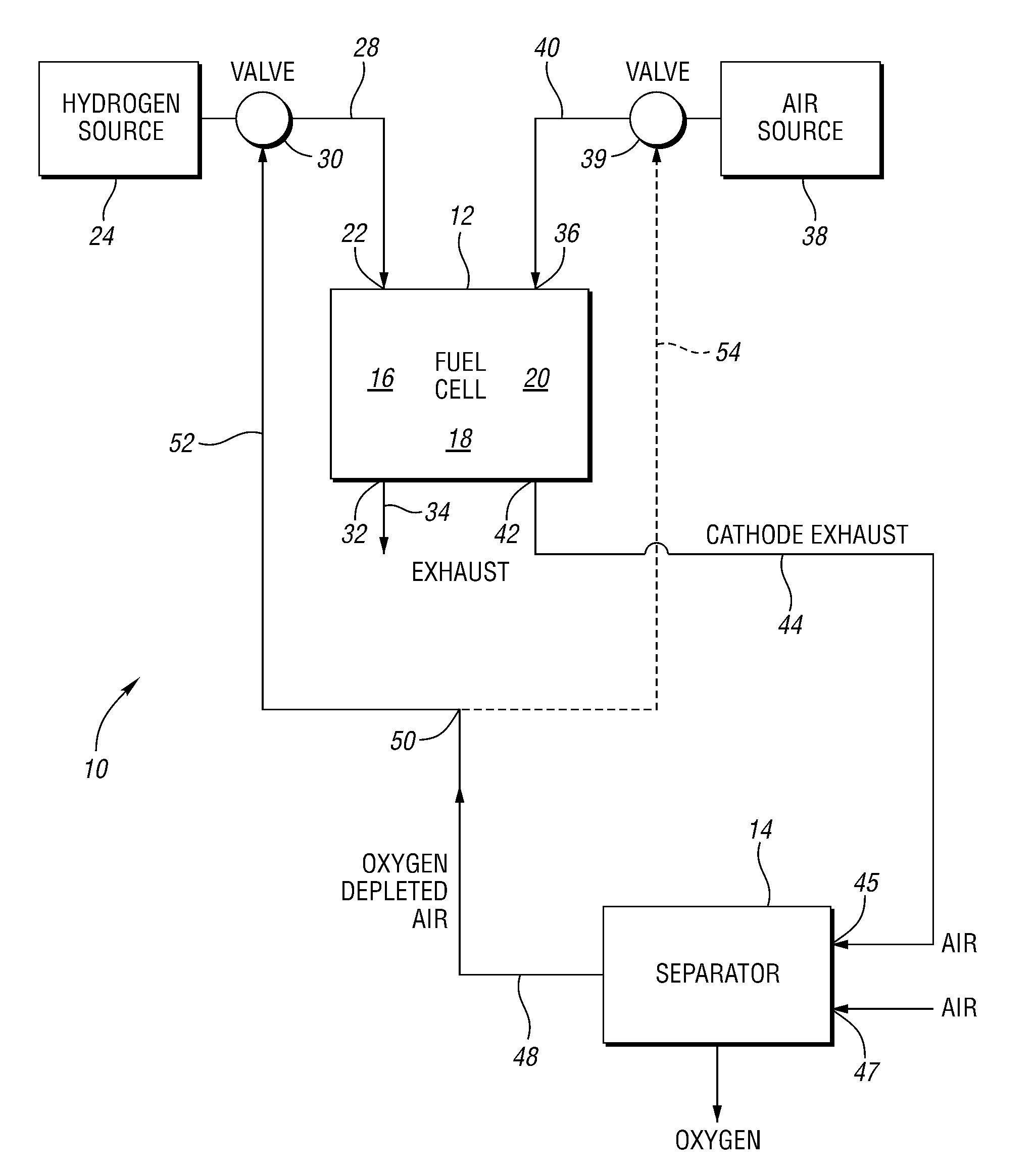
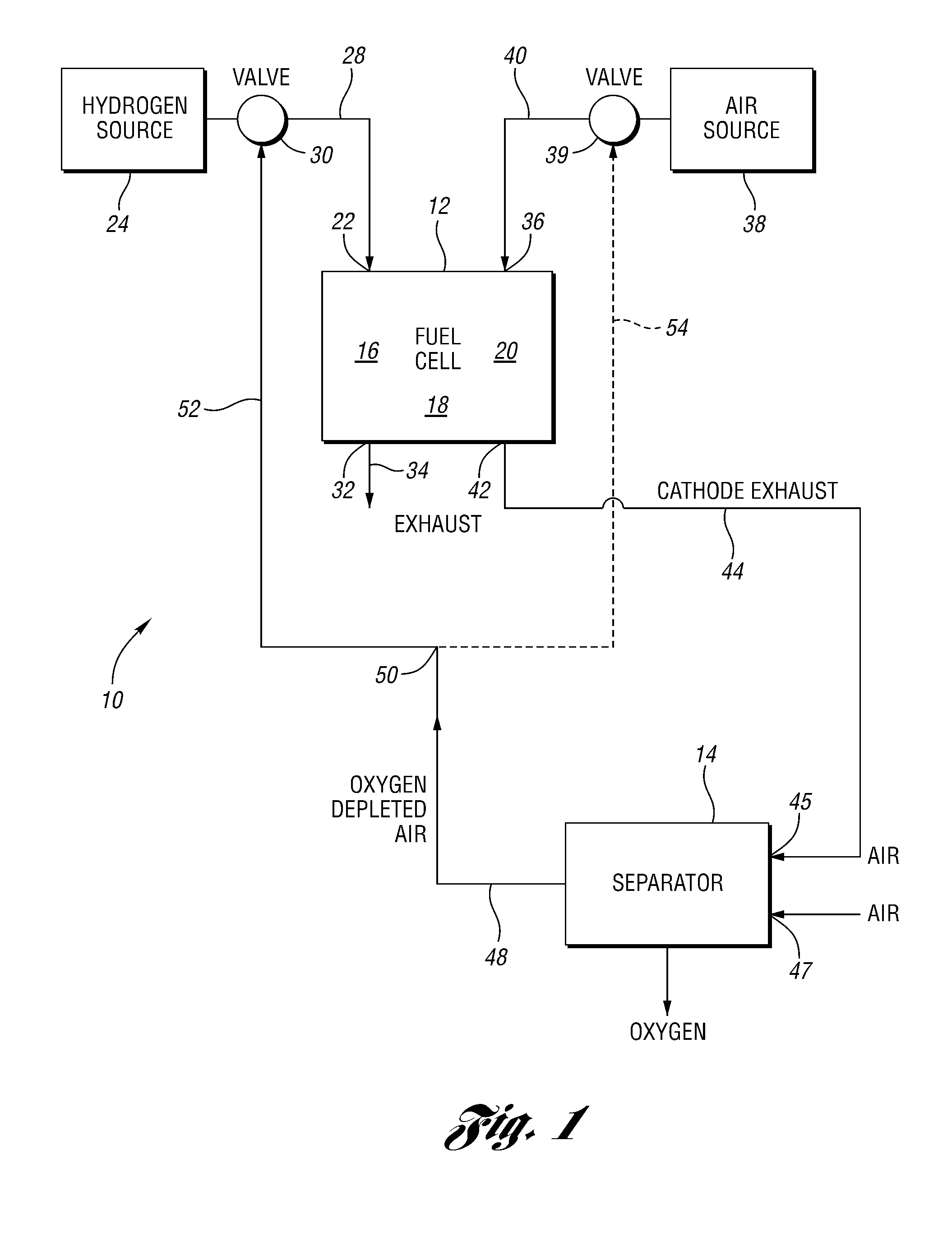
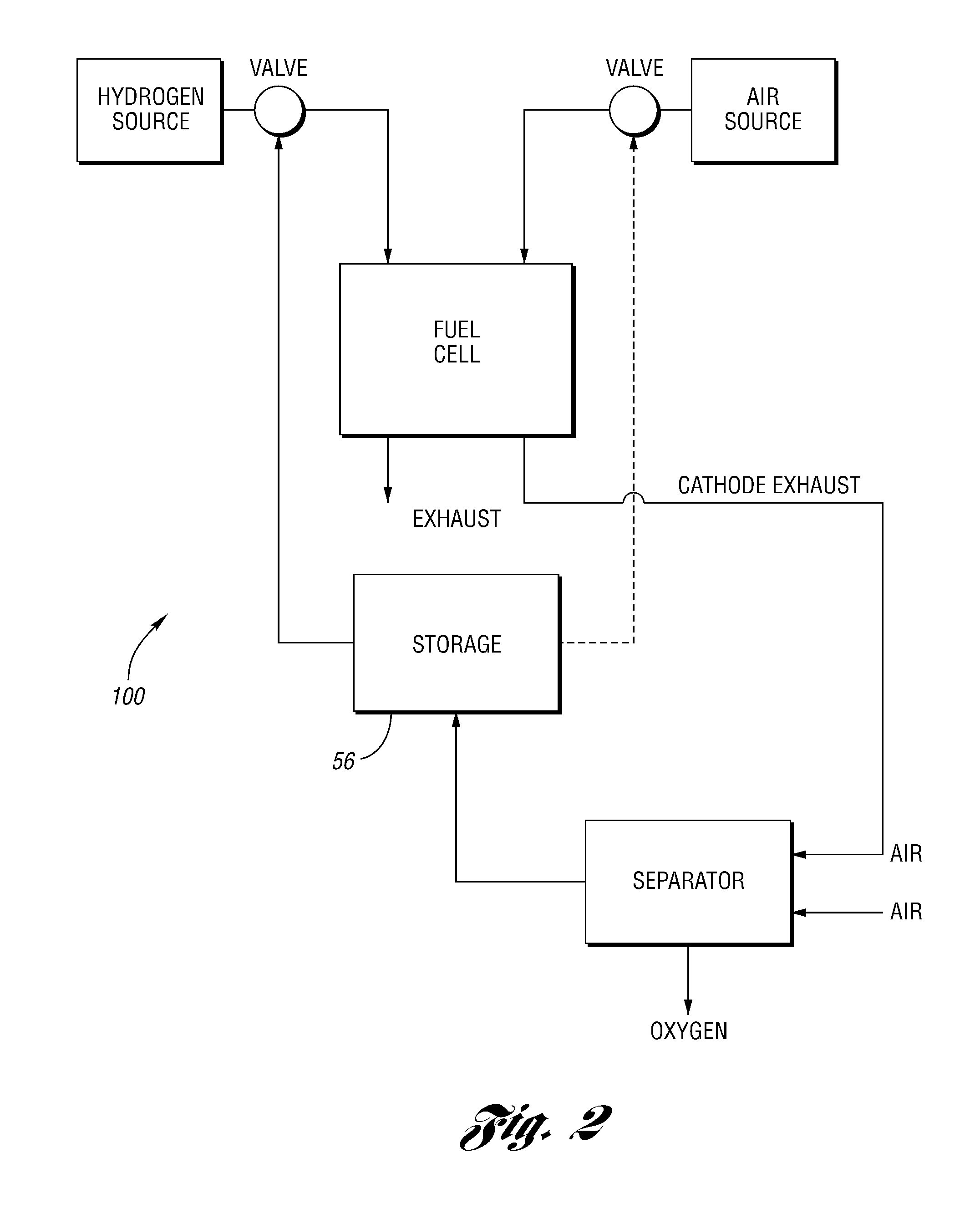
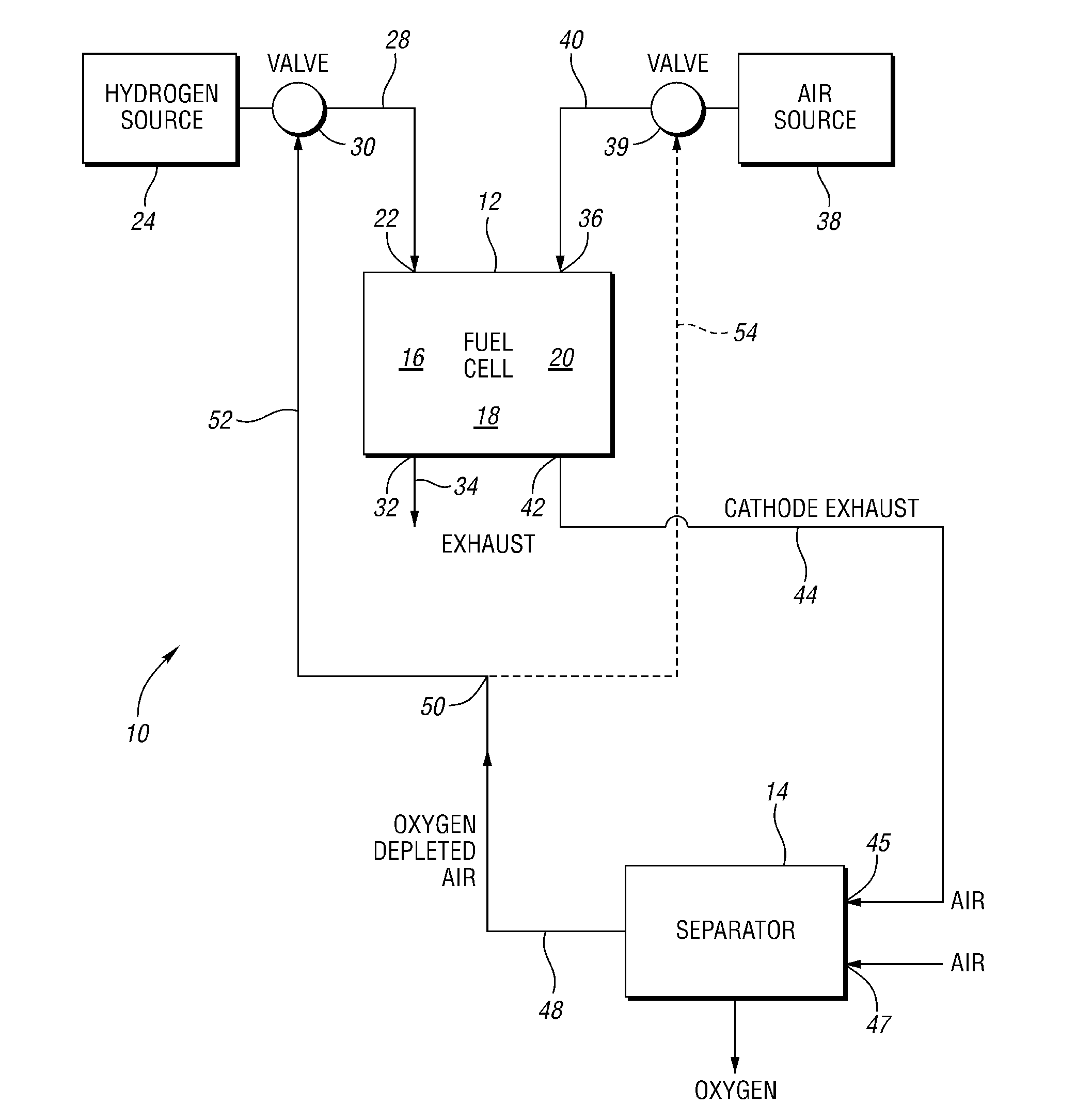
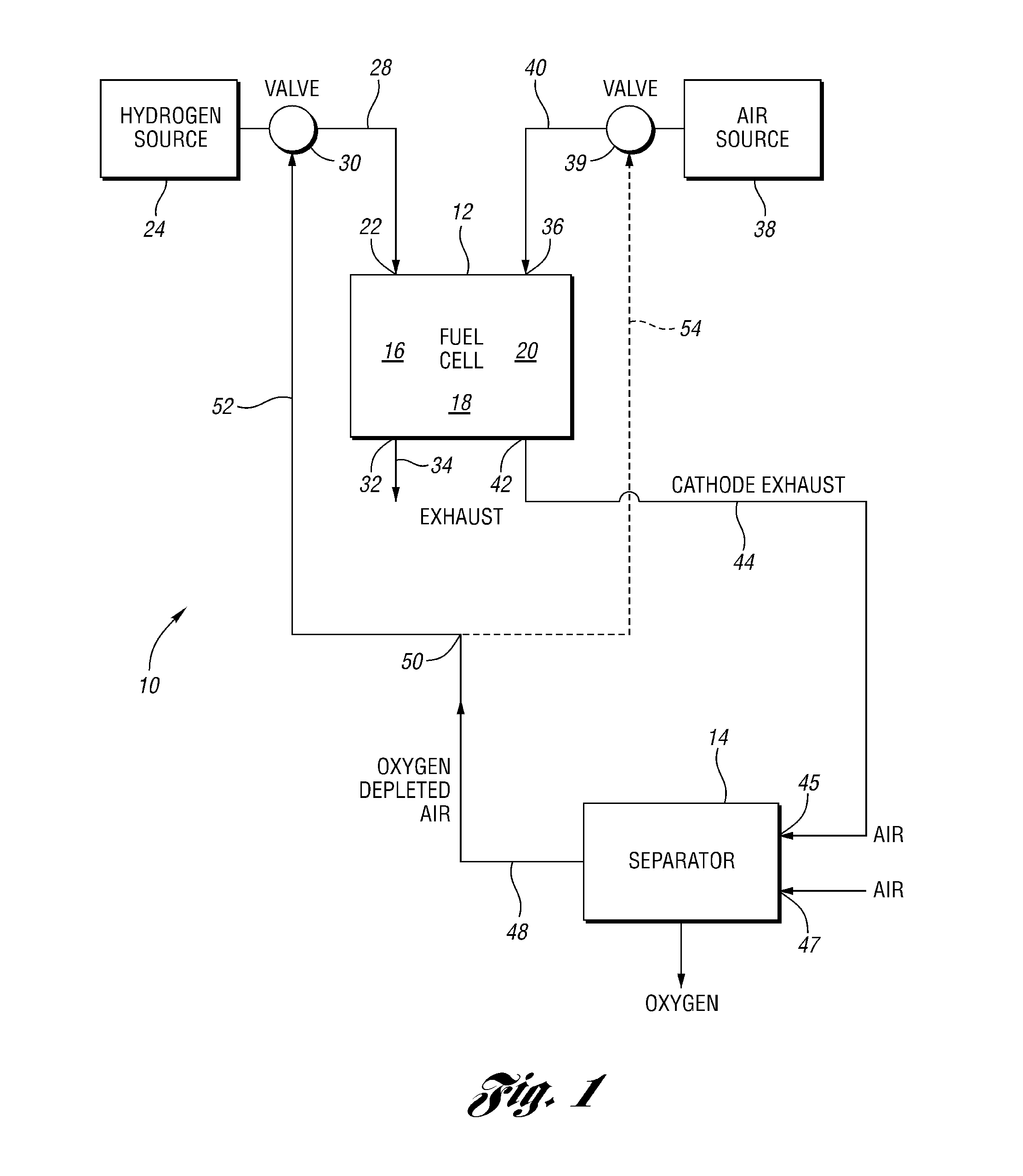
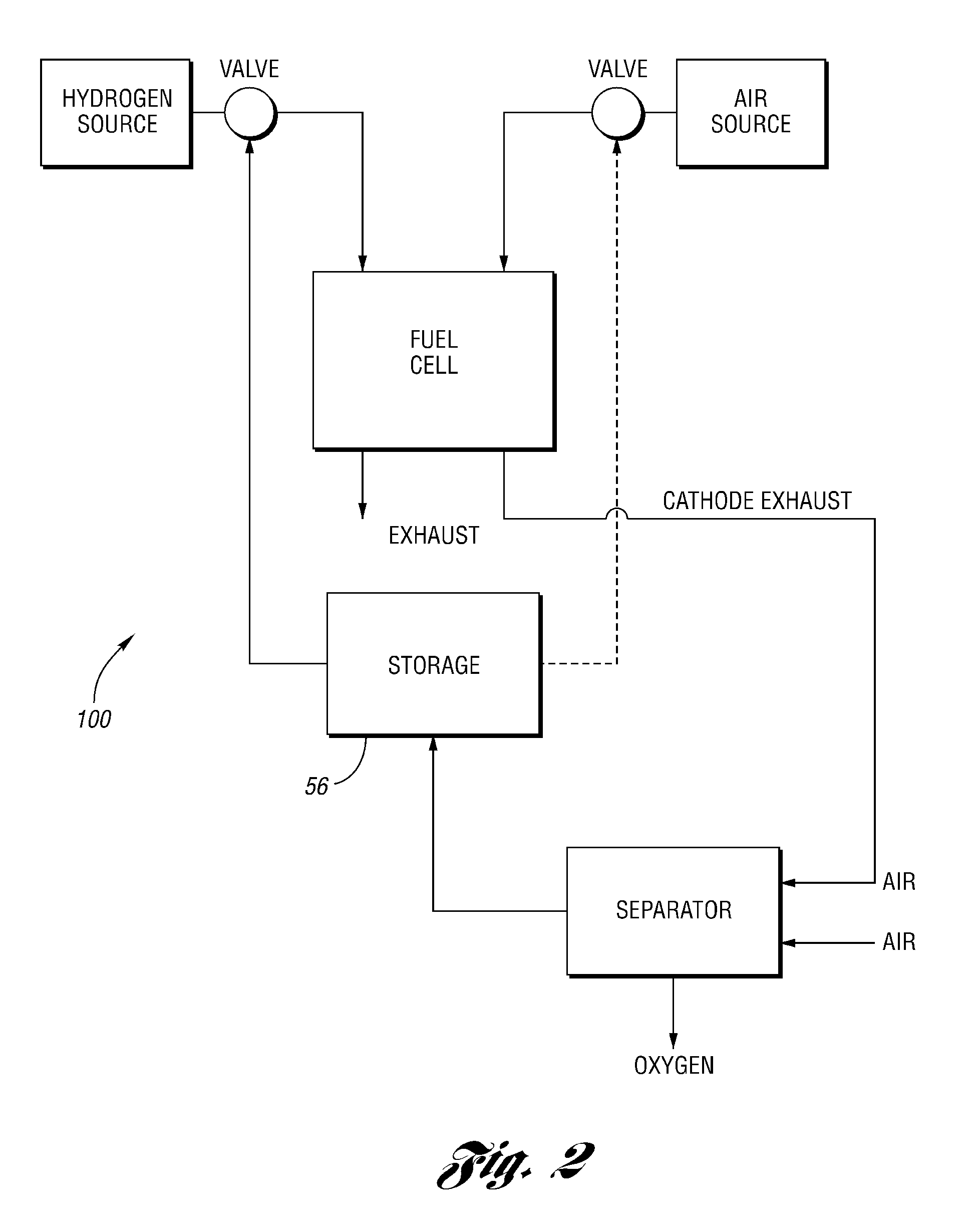
Oxygen removal systems during fuel cell shutdown
InactiveUS20090023040A1Minimize hydrogenMinimize oxygen mixingFuel cell auxillariesCell component detailsFuel cellsNitrogen
A purging system for removing oxygen from a fuel cell system during a shutdown period for the fuel cell system. The purging system includes a separator having an inlet and an outlet; a first exhaust line for communicating a first exhaust gas stream from an outlet of the fuel cell system to the separator inlet during the shutdown period of the fuel cell system; and a second exhaust line for communicating a second exhaust gas stream to an inlet of the fuel cell system for delivering the second exhaust gas stream to the fuel cell system during the shutdown period. The separator removes oxygen from the first exhaust gas stream such that the first stream nitrogen molar volume is lower than the second steam nitrogen molar volume and the first stream oxygen molar volume is higher than the second stream oxygen molar volume.
Owner:FORD MOTOR CO
Green high-efficiency recyclable SO2 gas absorbent and preparation thereof
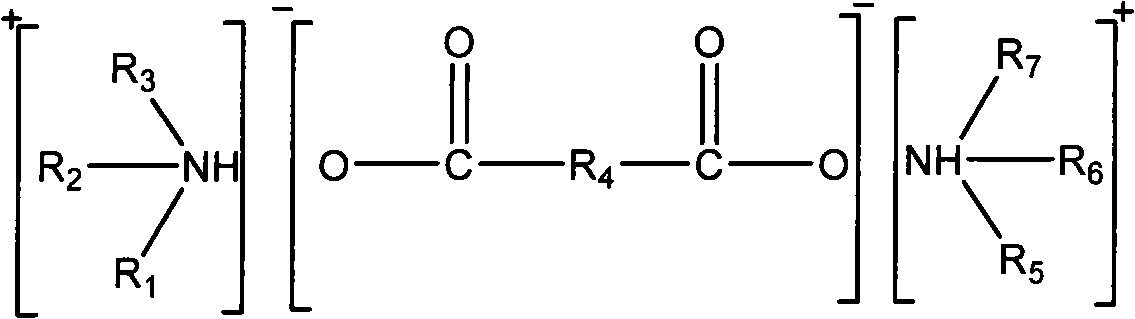
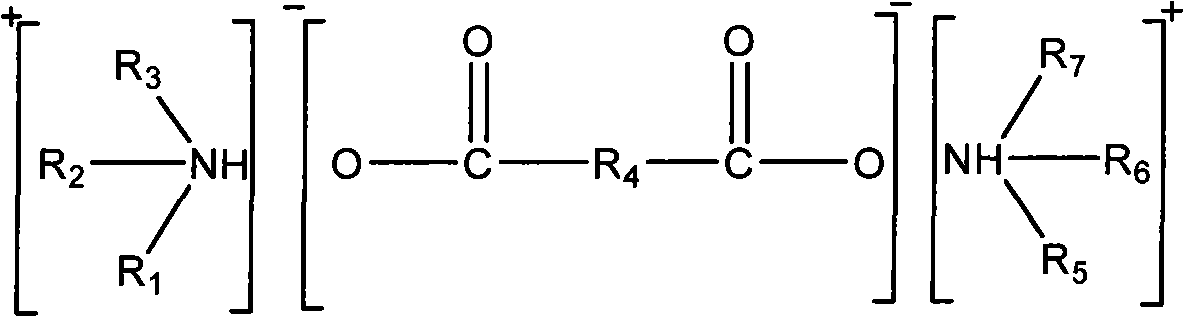
InactiveCN101264414AEasy to operateLower requirementDispersed particle separationAbsorption capacityHydrogen
The invention relates to a high-efficient and circulatory SO2 air absorbent and preparation method. The absorbent is an alkaline ionic liquid comprising dicarboxylate of amine shown by the following chemical formulas, wherein, R1, R2, R3, R5, R6, R7 from the alkyl of C1 - C8 or the replace alkyl or hydrogen (H), R4 from CnH2n-2 or the alkyl of CmH2m or the replace hydroxyl; wherein, n=2-8, m=0-8. The preparation method of the absorbent takes amine and dicarboxylate acid as raw materials and comprises a direct action in solvent or without solvent. The ratio of the absorption capacity of SO2 and absorbent molar volume is as high as 2:1; the SO2 can be desorbed for a plurality of times and can be recycled, and achieves rapid absorption and desorption balance; the desorption ratio is more than 90%.
Owner:HEFEI UNIV OF TECH
High-temperature hydrothermal synthesis method of phosphorus Al-Si SAPO-34
InactiveCN101531377AHigh yieldThe synthesis method is simpleMolecular-sieve and base-exchange phosphatesMolecular-sieve silicoaluminophosphatesStructural formulaOrganic compound
Owner:SHANGHAI SECOND POLYTECHNIC UNIVERSITY
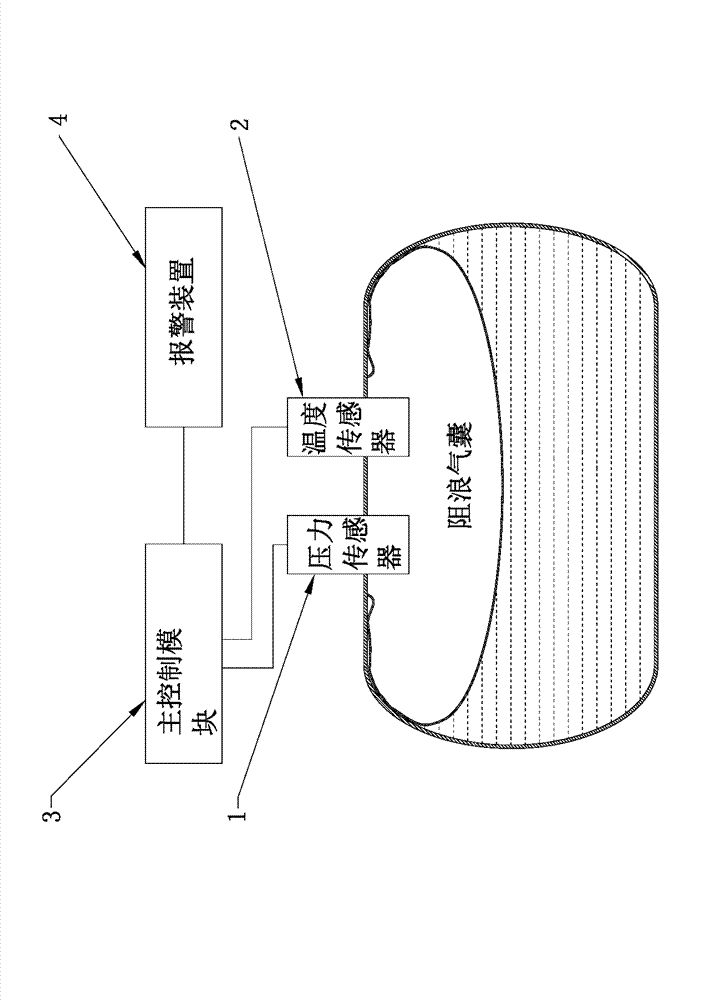
Wave-preventing air sac leakage detecting device and wave-preventing air sac leakage detecting system of tank truck
ActiveCN103115735AImprove reliabilityImprove effectivenessMeasurement of fluid loss/gain rateAir volumeEngineering
Owner:东莞市永强汽车制造有限公司
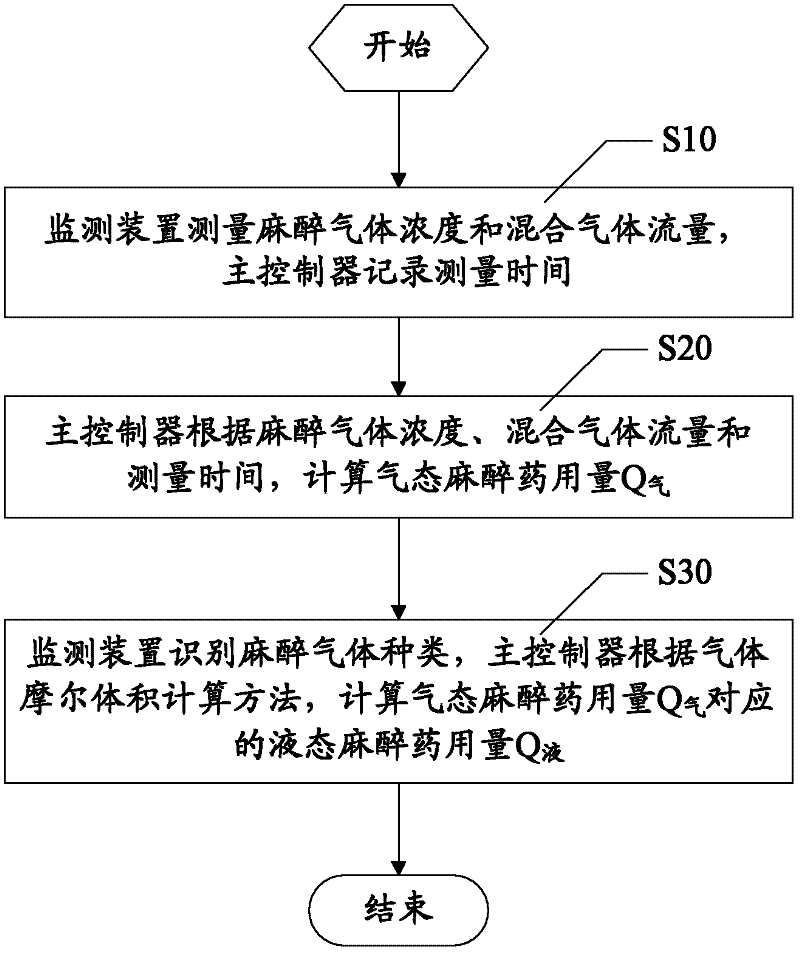
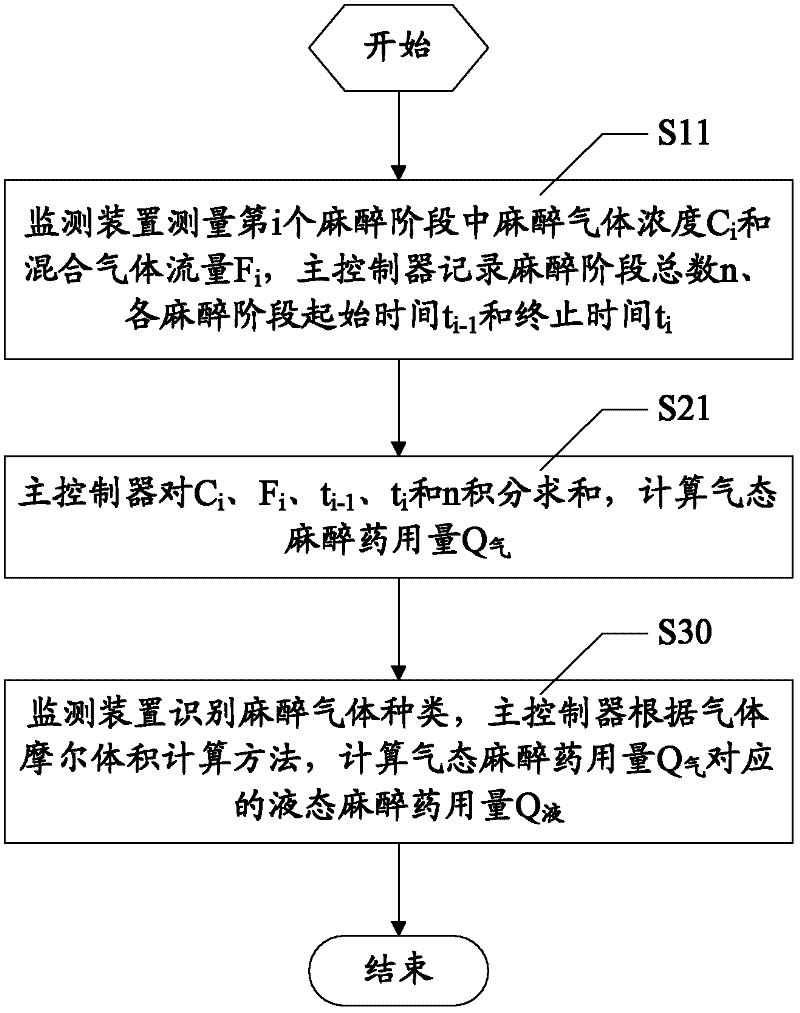
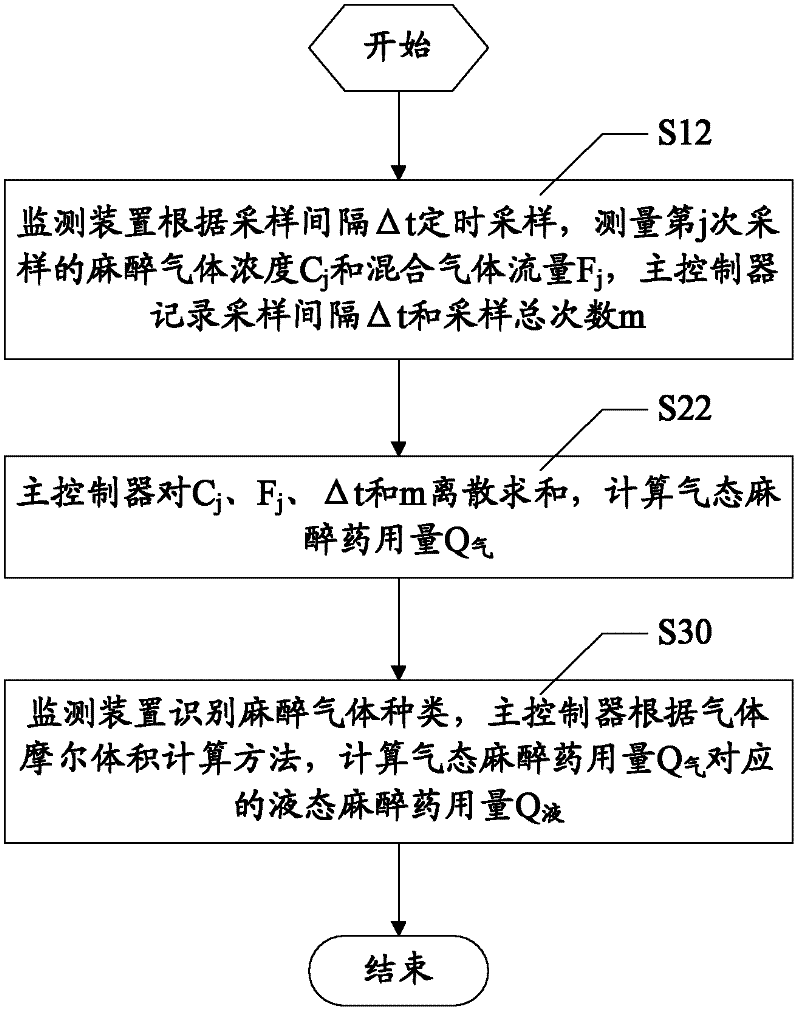
Anesthetic dosage calculation method and anesthetic dosage calculation system for anesthesia machine
ActiveCN102564521AImprove accuracyHigh precisionVolume measurement apparatus/methodsAnesthetic gasesGas concentration
The invention discloses an anesthetic dosage calculation method and an anesthetic dosage calculation system for an anesthesia machine. The method includes steps: using a monitoring device to measure anesthetic gas concentration and mixed gas flow, and using a main controller to record measuring time; using the main controller to calculate gaseous anesthetic dosage Q according to the anesthetic gas concentration, the mixed gas flow and the measuring time; and using the monitoring device to identify anesthetic gas types, and using the main controller to calculate liquid anesthetic dosage Q corresponding to the gaseous anesthetic dosage Q according to a gas molar volume calculation method. Errors caused by manual calculation can be avoided, accuracy and precision of anesthetic dosage calculation results can be improved effectively, and basis is provided for patient safety guaranteeing, operation costing and medical insurance claim.
Owner:深圳市普博医疗科技股份有限公司
Application of MIL-100 (Fe) in preparation of phenol through photocatalytic hydroxylation of benzene
InactiveCN104844423AReduce energy consumptionReduce pollutionOrganic chemistryOrganic compound preparationBenzeneEarth crust
The present invention discloses application of MIL-100 (Fe) in preparation of phenol through photocatalytic hydroxylation of benzene, and belongs to the field of photocatalytic technologies. MIL-100 (Fe) is a metal-organic framework material containing the Fe element that is high in abundance in the earth crust. According to the present invention, MIL-100 (Fe) is applied for the first time as a photocatalyst to preparation of phenol through hydroxylation of benzene; and the problems of high energy consumption, low selectivity and production of byproducts same in molar volume with the produced phenol in a three-step-method for phenol synthesization in the prior art are solved. The application of MIL-100 (Fe) in preparation of phenol through photocatalytic hydroxylation of benzene is simple in process, low in cost, and capable of meeting the practical production demands, and has a relatively great potential for application.
Owner:FUZHOU UNIV
Preparation method and application of load-type metal phthalocyanine catalyst
InactiveCN101804361ASimple methodLow costOrganic-compounds/hydrides/coordination-complexes catalystsWater/sewage treatmentVolumetric Mass DensityPhthalocyanine
The invention discloses a preparation method of load-type metal phthalocyanine catalyst, comprising: directly dipping a molecular sieve into phthalocyanine aqueous solution of which the molar volume density is 10-500 mu mol / L; adjusting the pH value to be 10-12; dipping at room temperature; stirring at interval; and centrifuging and drying to prepare the load-type metal phthalocyanine catalyst. The invention discloses an application of the catalyst in removing organic pollutants in water. The load-type metal phthalocyanine catalyst prepared with the invention has simple method, environment protection and low cost, the catalytic activity thereof is far higher than that of unloaded phthalocyanine catalyst, and the reaction condition is moderate and is not limited by waste water pH value. The load-type metal phthalocyanine catalyst does not generate secondary pollution, can be used for oxidation treatment of organic waste water systems and has industrial application prospect.
Owner:ZHEJIANG UNIV
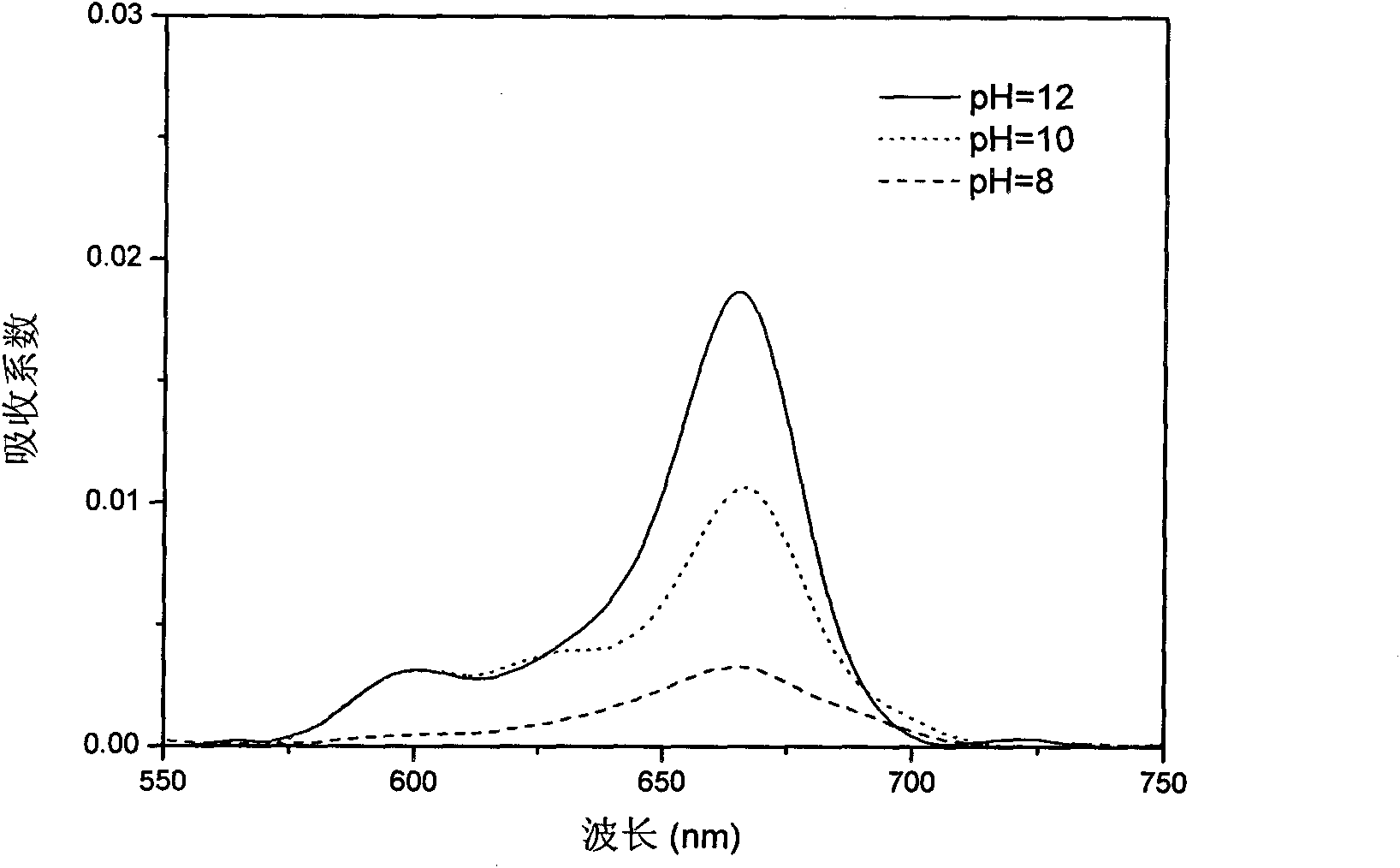
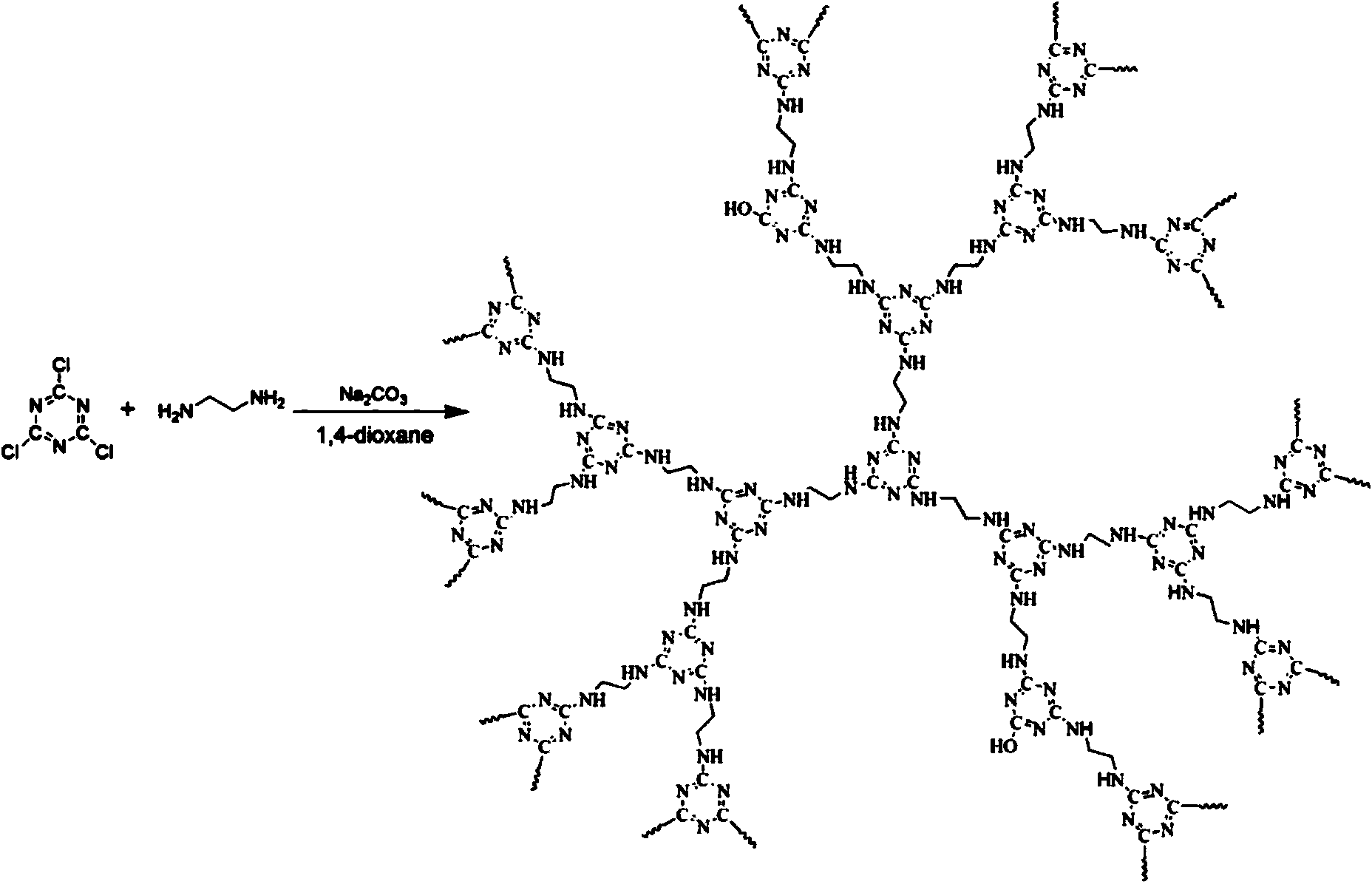
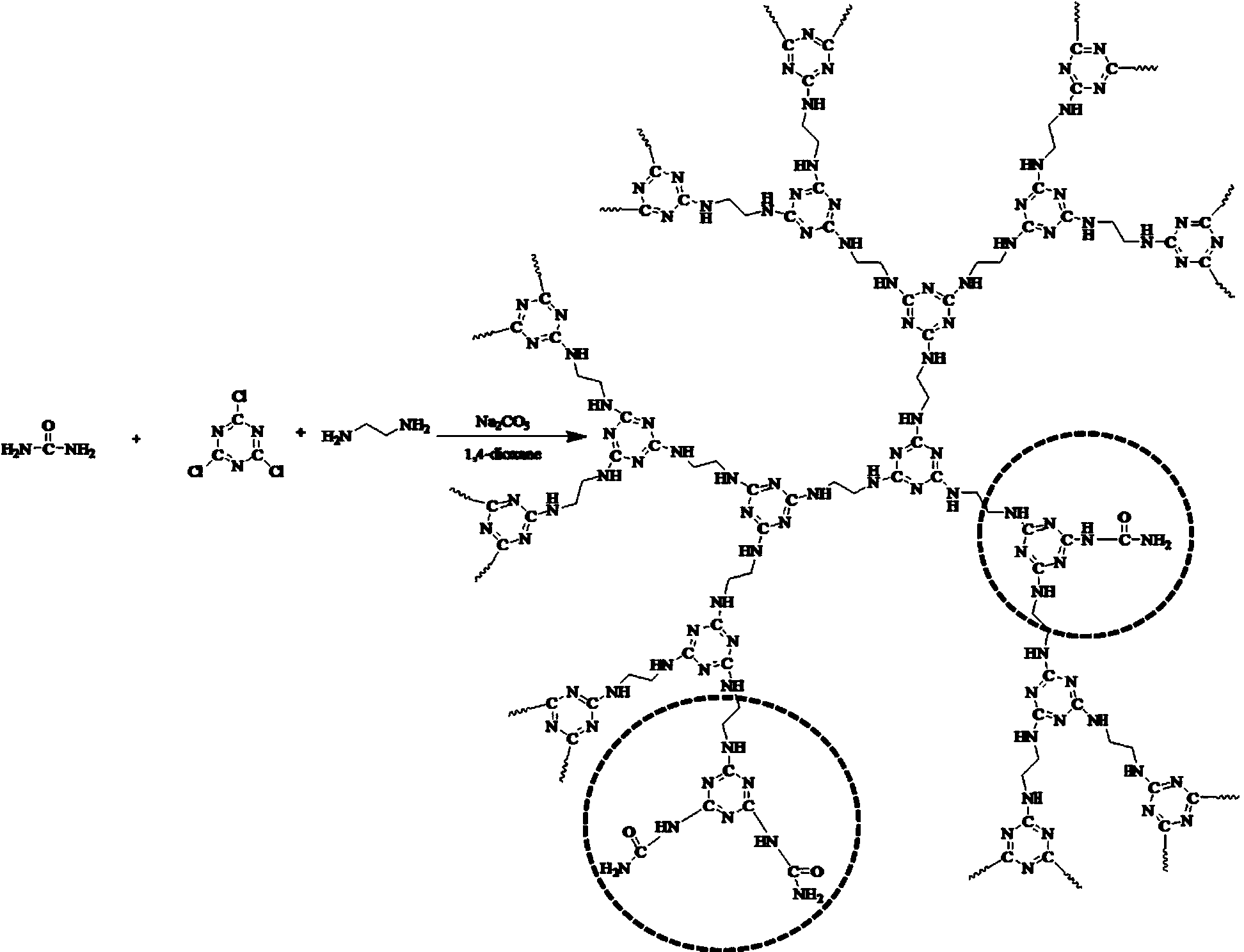
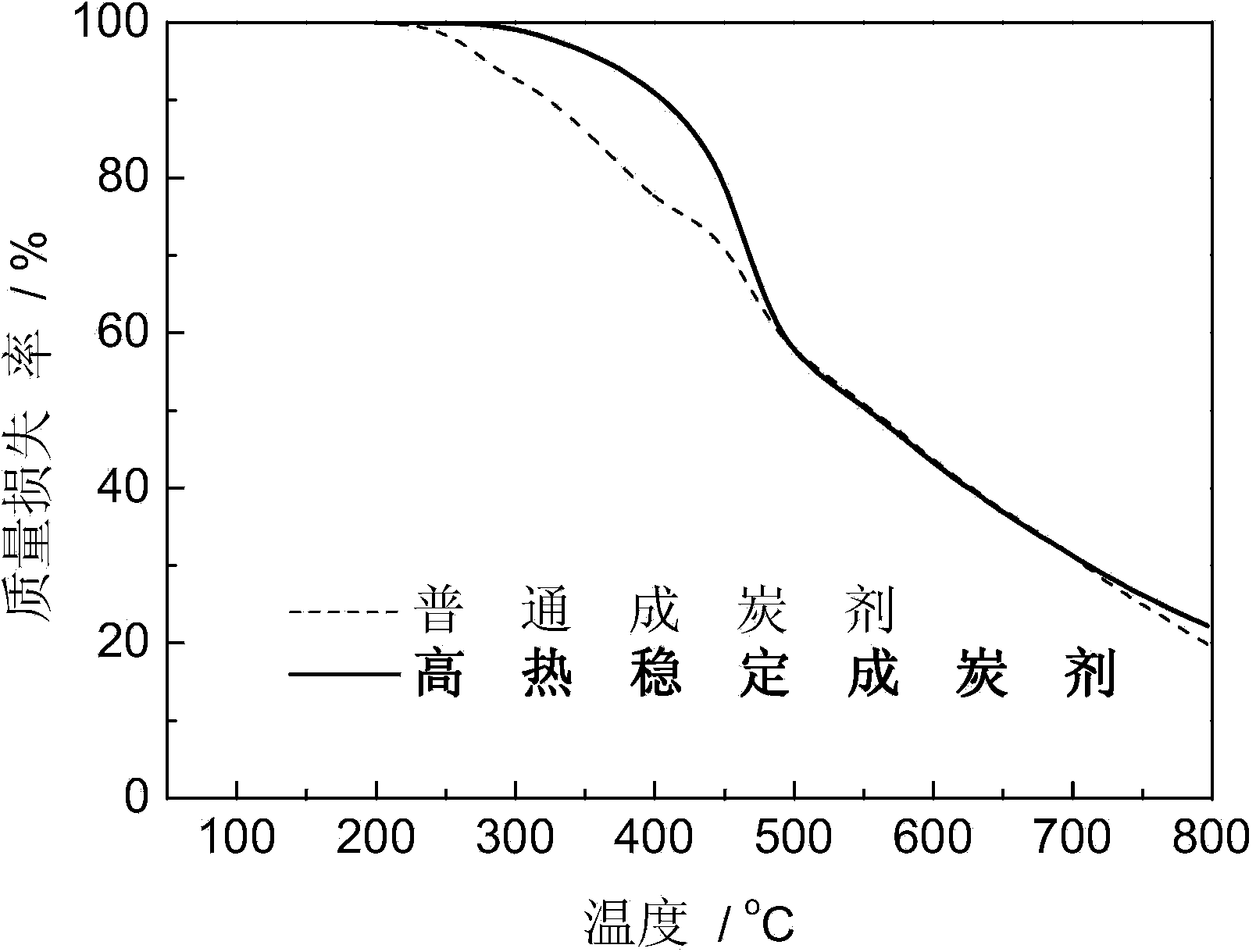
High-heat-stability charring agent and preparation method thereof
The invention relates to the field of charring agents, and particularly relates to a high-heat-stability charring agent and a preparation method thereof. The high-heat-stability charring agent is prepared from the following raw materials: cyanuric chloride, an acid-binding agent, a nitrogen-containing compound, ethylenediamine and an organic solvent, wherein the nitrogen-containing compound is one of urea, aqua ammonia and ammonium carbonate; the molar ratio of the cyanuric chloride to the acid-binding agent is 1: (3.0-3.5), the molar ratio of the cyanuric chloride to the ethylenediamine is 1: (1-2), the molar ratio of the nitrogen-containing compound to the ethylenediamine is 1: (4-19), and the molar volume ratio of the cyanuric chloride to the organic solvent is 0.4-0.6 mol / L. The high stability of the high-heat-stability charring agent provided by the invention is significantly increased, molecular chains of an obtained charring agent are uneasily entangled, and the charring agent is high in productivity. The preparation method is simple and practicable, low in cost, high in yield, and less in produced wastes, and the obtained high-heat-stability charring agent has extremely good high heat stability, thereby facilitating the wide application of the high-heat-stability charring agent.
Owner:BEIJING TECHNOLOGY AND BUSINESS UNIVERSITY
Clean and efficient gas fire extinguishing agent composition
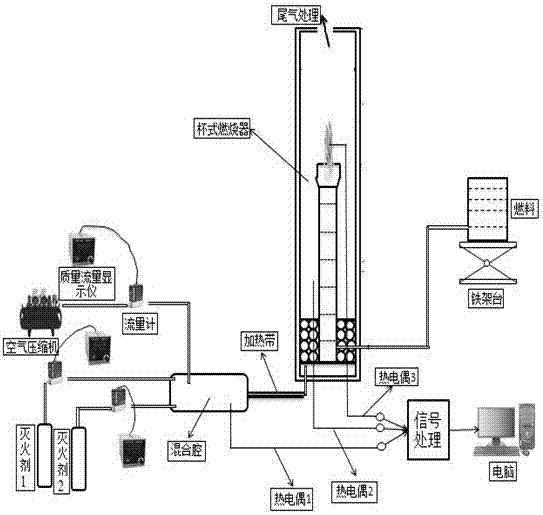
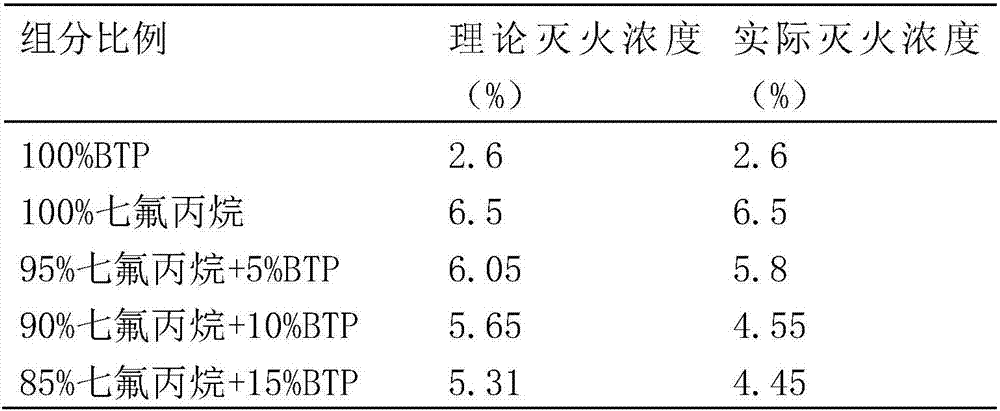
InactiveCN107213576AIncrease fire extinguishing concentrationReduce storage volumeFire extinguisherBromineNitrogen
A clean and efficient gas fire extinguishing agent composition comprises, with the molar volume as the benchmark, 5%-15% of 2-bromine-3,3,3-trifluoropropene (BTP) and 95%-85% of a hydrofluoroalkane fire extinguishing agent or an inert gas fire extinguishing agent. The hydrofluoroalkane fire extinguishing agent comprises one or more of heptafluoropropane, pentafluoroethane, trifluoromethane and 1,1,2,2,3,3,4-heptafluorocyclopentane. The inert gas fire extinguishing agent comprises one or more of nitrogen and carbon dioxide. By the adoption of the gas fire extinguishing agent composition, the fire extinguishing concentration of inert gas can be increased, the storage capacity is reduced, the global warming potential of hydrofluoroalkane is lowered, the environment is improved, the fire extinguishing effect is enhanced, the property is stable, no precipitation is generated after long-time storage, the raw materials are obtained easily, and the production cost is low.
Owner:JIUJIANG CSSC CHEM TECH +1
Polyimide nano-foam and preparation method thereof
The invention provides polyimide nano-foam and a preparation method thereof .The preparation method includes the following steps that firstly, a diamine compound containing admantyl groups, a diamine compound containing no admantyl group and a dianhydride compound are subjected to a polymerization reaction in a solvent, and a polyamide acid solution is obtained; secondly, a compound with thermal instability and the polyamide acid solution are subjected to uniform dispersion or a polymerization reaction, tape casting, curing and thermal imidization in sequence, and a polyimide film is obtained; thirdly, the polyimide film is subjected to thermal decomposition, and the polyimide nano-foam is obtained .As the admantyl groups are large-size groups, the molar volume of atomic groups is increased, then the dielectric constant of a polyimide matrix is effectively reduced, and accordingly the dielectric constant of the polyimide nano-foam material is reduced; besides, by introducing the admantyl groups, the glass-transition temperature of polyimide is increased, and the thermal stability of the material is improved .Moreover, the polyimide nano-foam has good mechanical properties.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Copper and Schiff base complex and preparation method and application thereof
ActiveCN107827914AAvoid pollutionSimple preparation processAntibacterial agentsGroup 1/11 organic compounds without C-metal linkagesEscherichia coliSalicylaldehyde
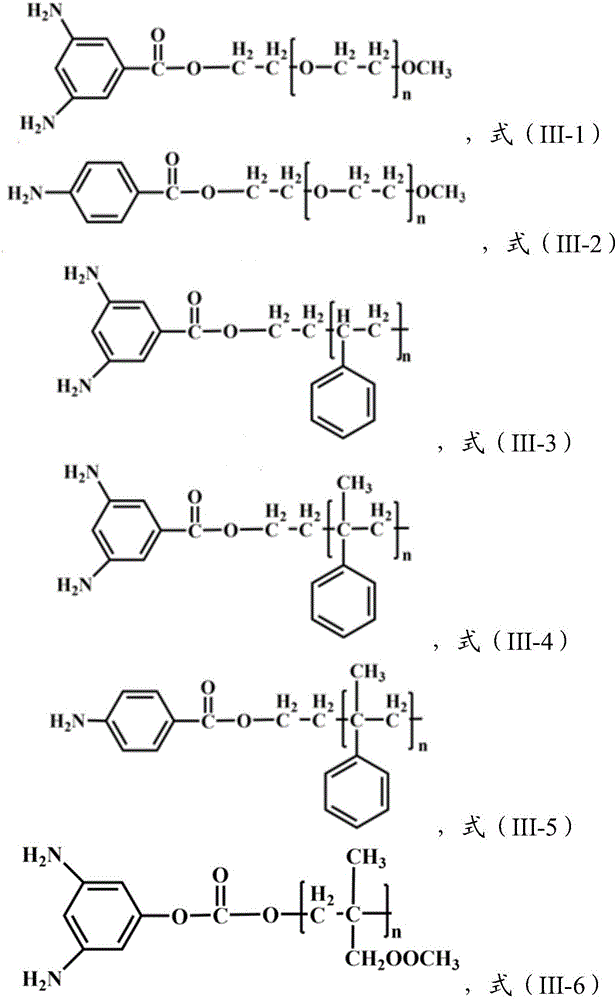
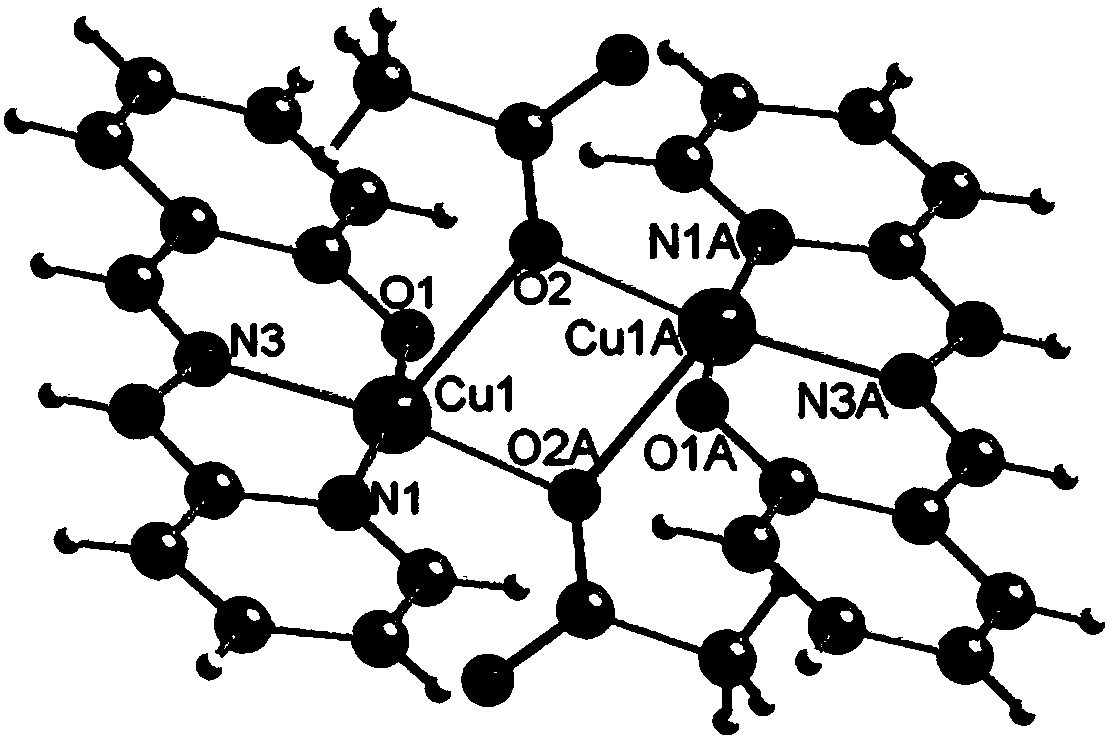
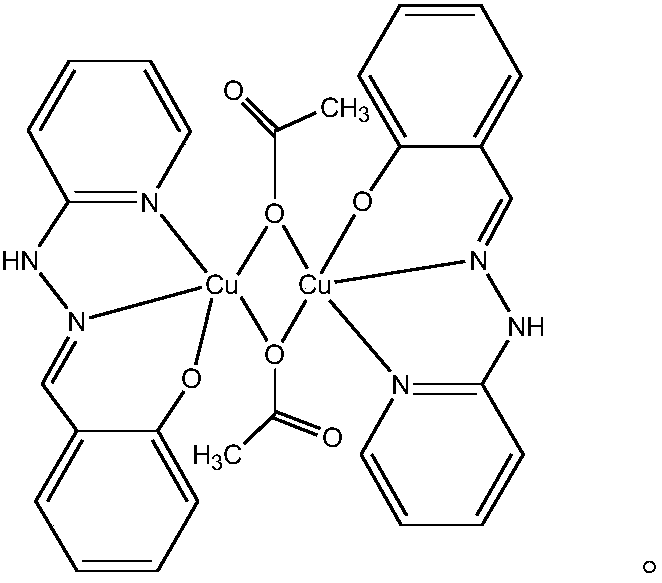
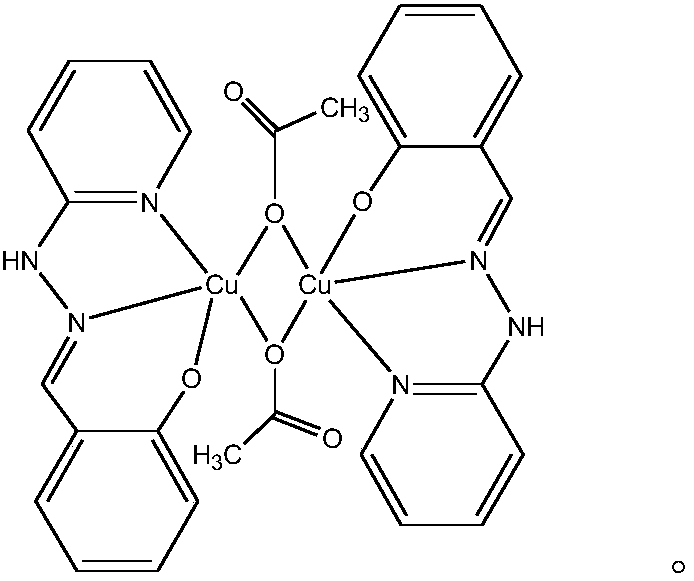
The invention relates to a copper and Schiff base complex and a preparation method and application thereof, and aims to solve the technical problems that a conventional copper and Schiff base complexpreparation method is troublesome in operation, long in reaction time and low in productivity and has potential safety hazards. The copper and Schiff base complex has the molecular formula [Cu2(HL)2(OAc)2]n, wherein HL is diazanyl-(2-pyridyl)salicylaldimine. The preparation method of the copper and Schiff base complex comprises the following steps: adding Cu(OAc)2.H2O, 2-hydrazinylpyridine and salicylaldehyde of which the molar ratio is 1:2:2 into a polytetrafluoroethylene tube containing methyl alcohol, wherein the molar volume ratio of Cu(OAc)2.H2O to methyl alcohol is 0.1:(3-5) mmol / ml; performing a reaction for 48-72 h at 80-100 DEG C; performing natural cooling to separate out black rhabdolith; and after washing with a methanol solution, performing drying to prepare the copper and Schiff base complex. The copper and Schiff base complex has a powerful bacterial inhibition function on escherichia coli, staphylococcus aureus and bacillus subtilis, has a favorable potential application prospect in the field of biological activity, and can serve as a novel antibacterial agent.
Owner:SHANXI UNIV
Rhodamine B derivatives-based multi-channel fluorescent probe as well as preparation method and application thereof
ActiveCN107652299ASimple methodEasy to operateOrganic chemistryFluorescence/phosphorescenceDistillationFluorescence
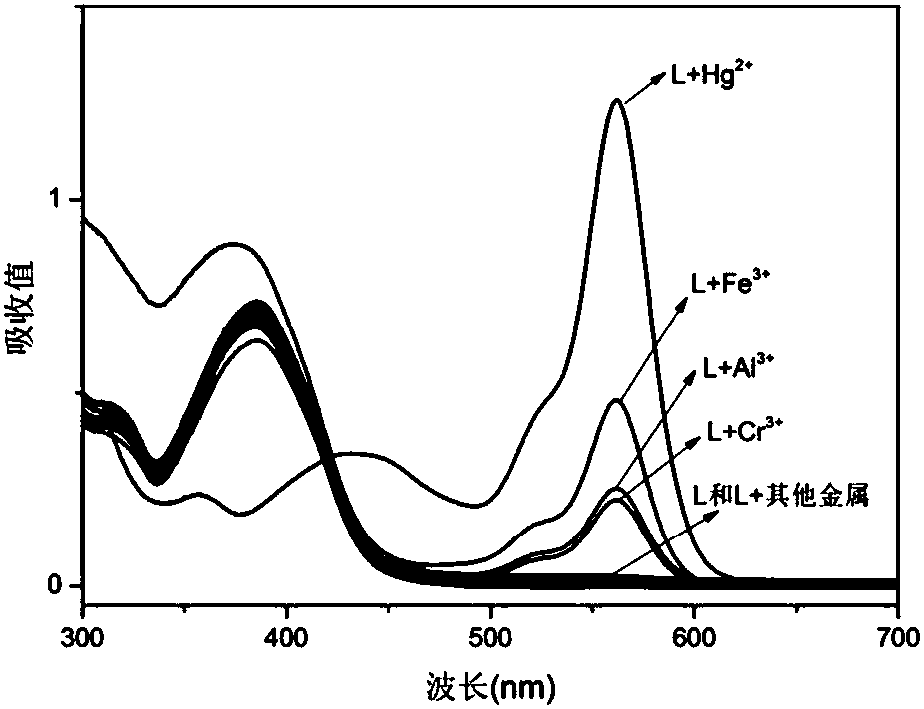
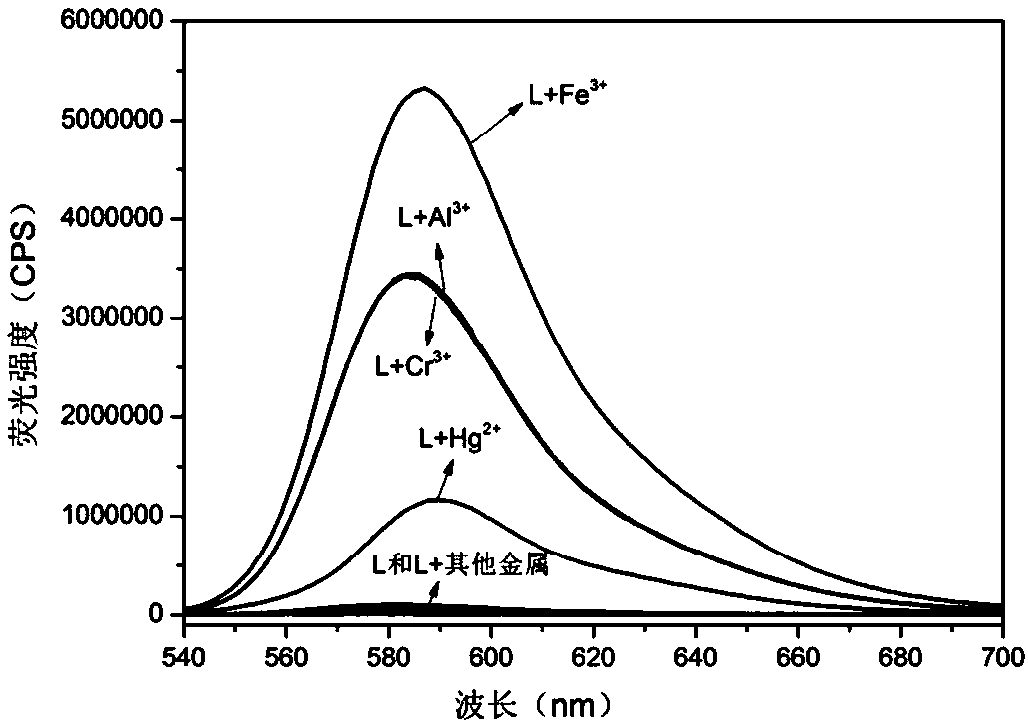
The invention discloses a rhodamine B derivatives-based multi-channel fluorescent probe, a preparation method and application thereof. The chemical formula of the fluorescent probe is C34H34N8O2; thepreparation method includes steps of preparing a compound A by mixing rhodamine B and hydrazine hydrate in accordance with a molar ratio of (0.25-1):1; then preparing a compound B by mixing the compound A and glyoxal in accordance with the molar ratio of (0.25-1):1; dissolving the compound B and diaminomaleonitrile in anhydrous ethanol in accordance with the molar ratio of (0.25-1):1; adding 2-3 drops of glacial acetic acid and preparing the multi-channel fluorescent probe through stirring, refluxing, distillation and separation. The fluorescent probe is applied to detection of trivalent metalion and Hg<2+> ion. Furthermore, the invention has the significant advantage that the fluorescent probe can enhance recognition of Fe<3+>, Cr<3+>, Al<3+> and Hg<2+> fluorescence in identical solventenvironment, with high detection sensitivity.
Owner:SOUTHEAST UNIV
Method for providing robustness to the solution of the cubic equation of state
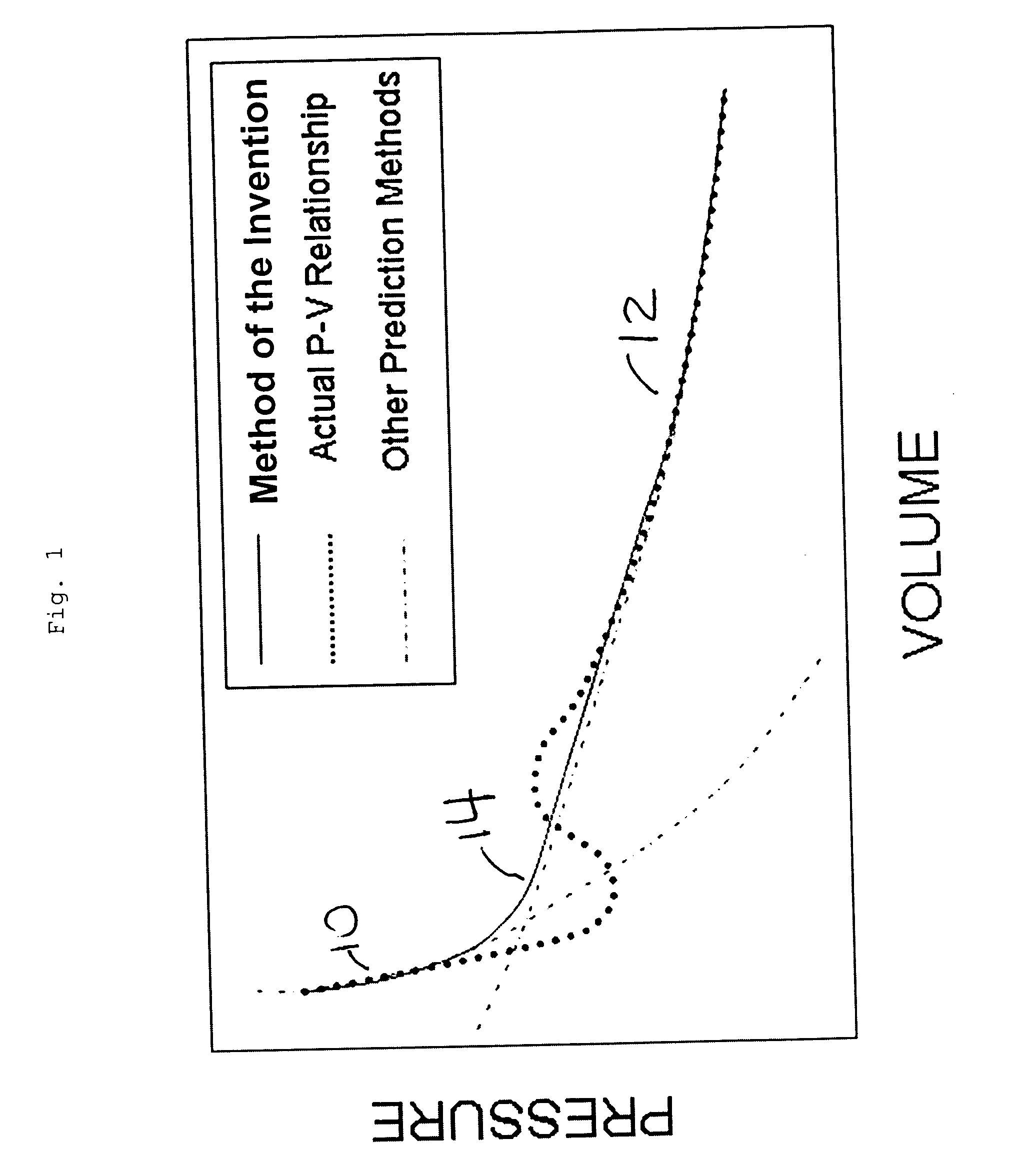
ActiveUS20050010629A1Fluid pressure measurementSpecial data processing applicationsOperant conditioningPhysical property
The cubic equations of state (CEOS) relate pressure, temperature and molar volume of a mixture. The deviation of the physical properties of a mixture from ideality is expressed by the compressibility factor. The compressibility factor is calculated from the CEOS. There is described a solution to the CEOS which provides a continuous solution for compressibility factors over the entire range of operating conditions. This solution avoids the non-unique roots, trivial roots and singularities that are present in the prior art solutions of the CEOS.
Owner:ABB INC
Normal-temperature storing liquid for intestinal flora sample as well as preparation method and application of normal-temperature storing liquid
InactiveCN108192827AEasy to transport at room temperatureMicroorganism based processesMicroorganism preservationEthylene diamineAcetic acid
The invention belongs to the field of molecular biologic intestinal flora, and specifically relates to normal-temperature storing liquid for an intestinal flora sample as well as a preparation methodand application of the normal-temperature storing liquid. The normal-temperature storing liquid comprises the following components: 0.02-0.1 % by mass of Kathon preservative, 1-10 mM by mole of ethylene diamine tetraacetic acid, 5-20 mM by mole of Tris-HCl buffering solution, 2mol / L by mole of lithium chloride, 95% by volume of ethyl alcohol, and 0-3% by volume of sterile water. The storing liquidis applicable to storing and conveying of intestinal flora sample under normal temperature; the sampled intestinal flora sample is soaked in the storing liquid; the stability of the microorganism structure in the sample can be maintained under normal temperature. Therefore, the sample collecting and storing of a subject can be performed in any time; meanwhile, the sample is conveniently conveyedin normal temperature.
Owner:深圳谱元科技有限公司
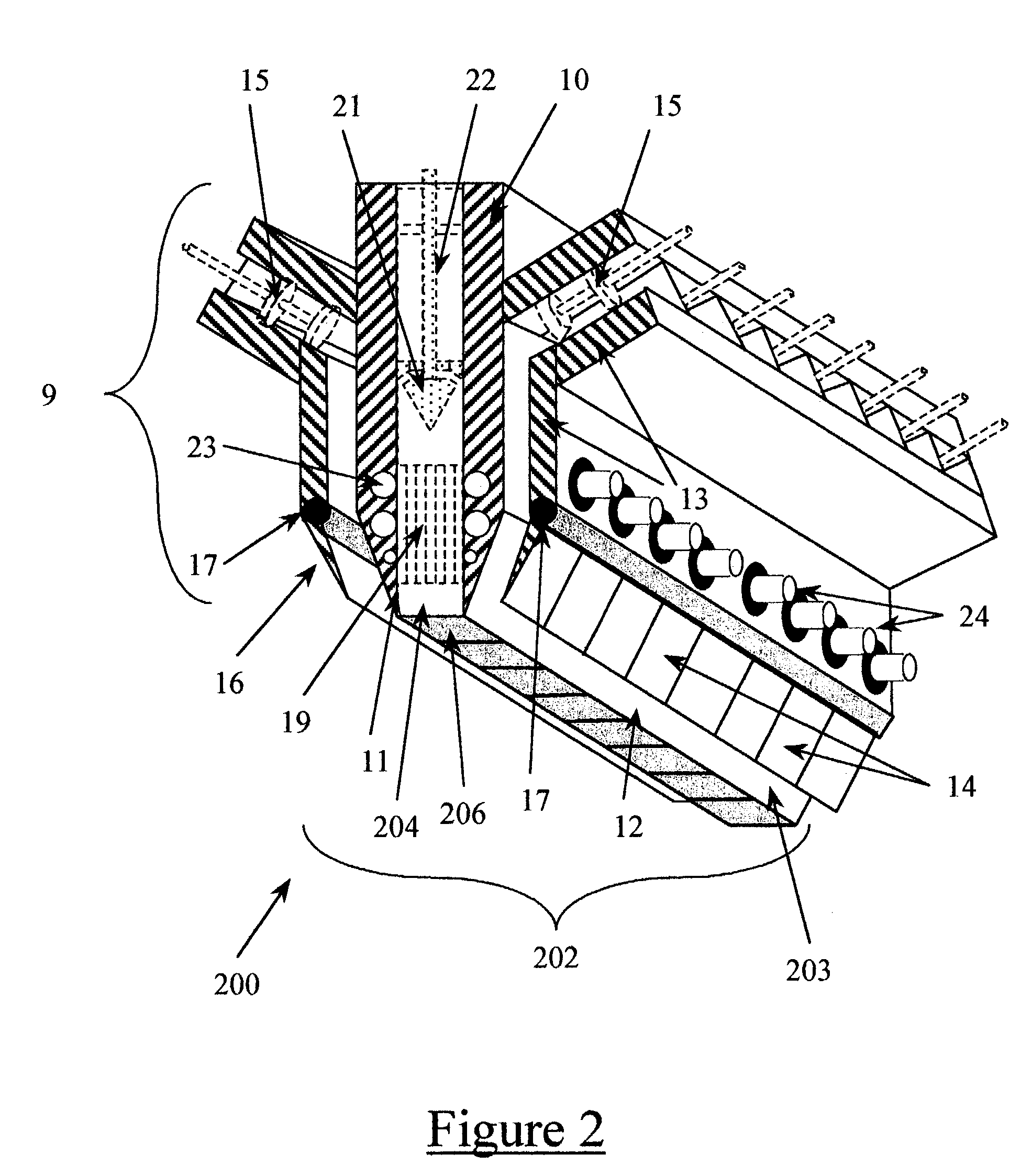
Process for preparing a diamond substance
A process for producing a diamond substance with a first inner nozzle and a second outer nozzle. In the first step of the process, a first mixture comprised of oxygen and a hydrocarbon gas is formed in the first inner nozzle; such hydrocarbon gas contains from about 1.01 to about 1.1 moles of carbon for each mole of oxygen present in such first mixture, and said first mixture contains at least about 10 volume percent of hydrocarbon gas. In the second step of the process, the first mixture is ignited to produce a flame core. In the third step of the process, a second mixture comprised of hydrogen and oxygen is formed in the outer nozzle; the second mixture is comprised of at least 2 moles of said hydrogen for each mole of said oxygen present in the second mixture; hydrogen gas and oxygen gas comprise at least about 20 molar volume percent of the second mixture; and the second mixture contains up to about 5 volume percent of hydrocarbon gas. In the fourth step of the process, the second mixture is ignited to produce a flame sheath. The flame sheath is disposed around the flame core so that the flame sheath completely surrounds said flame core and completely shields said flame core from the ambient atmosphere, thereby-producing a composite flame; and the composite claim is contacted with a substrate.
Owner:WOJAK GREGORY J
Method for preparing 4,4,5,5-dinaphthalene tetracarboxylic acid dianhydride
InactiveCN101397287AImprove efficiencyReduce usageOrganic chemistryNaphthalenetetracarboxylic dianhydridePotassium hydroxide
The invention relates to a preparation method widely used for preparing a 4, 4', 5, 5'-binaphthyl-tetracarboxylic dianhydride monomer of polyimide materials. The method is as follows: 4-halo-1, 8-anhydride naphthalene and potassium hydroxide which is 4 to 10 times of the molar volume of the 4-halo-1, 8-anhydride naphthalene are added with distilled water and heated for dissolving; then a Pd / C catalyst and a reducer are added, the temperature of a reaction system is controlled in a range between 80 and 120 DEG C and the reflux reaction is carried out for 3 to 40 hours; the Pd / C and impurities are filtered out and then hydrochloric acid or nitric acid is added into the filtrate for acidification so as to lead the pH value of the reaction system to be 1 to 3 and obtain yellow emulsion precipitation; the precipitation is washed by distilled water for 3 to 5 times and then is dried in a vacuum oven for 10 to 20 hours at the temperature of 180 to 200 DEG C so as to obtain yellow powder solids; then DMF is adopted for recrystallization so as to obtain the 4, 4', 5, 5'-binaphthyl-tetracarboxylic dianhydride monomer. The method of the invention is characterized by simple and convenient operation, being practical and having good effect.
Owner:JILIN UNIV
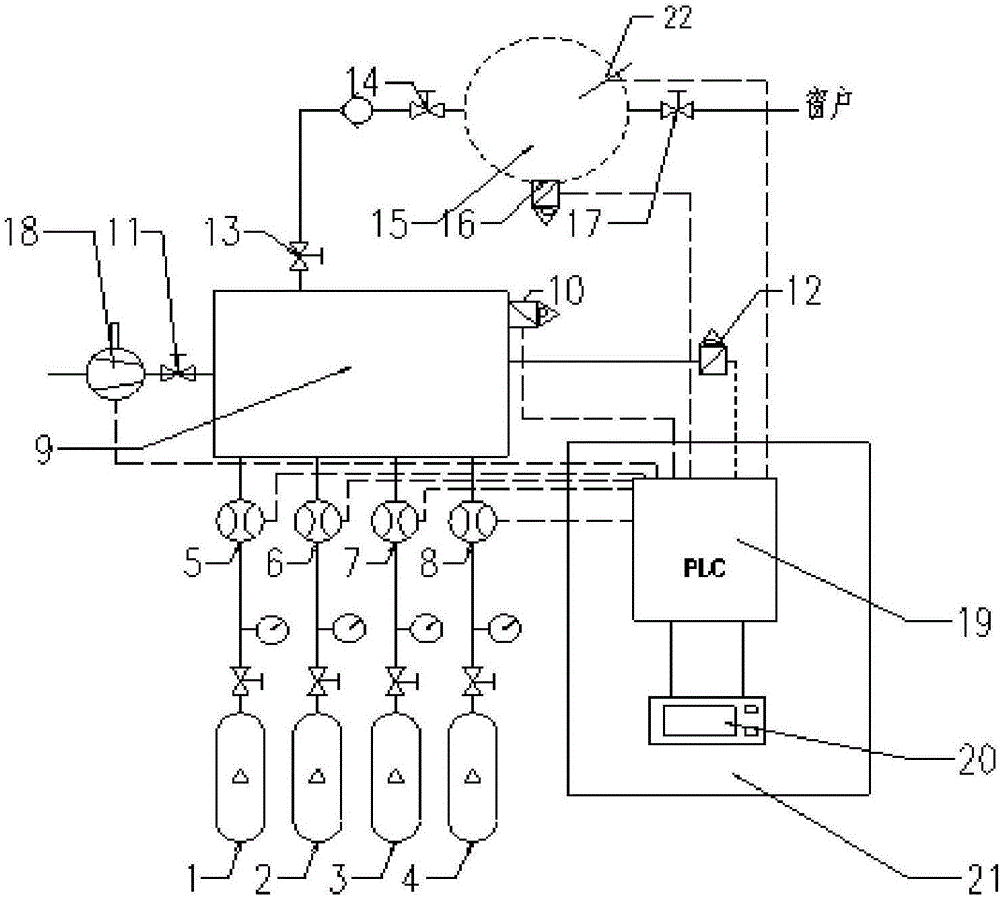
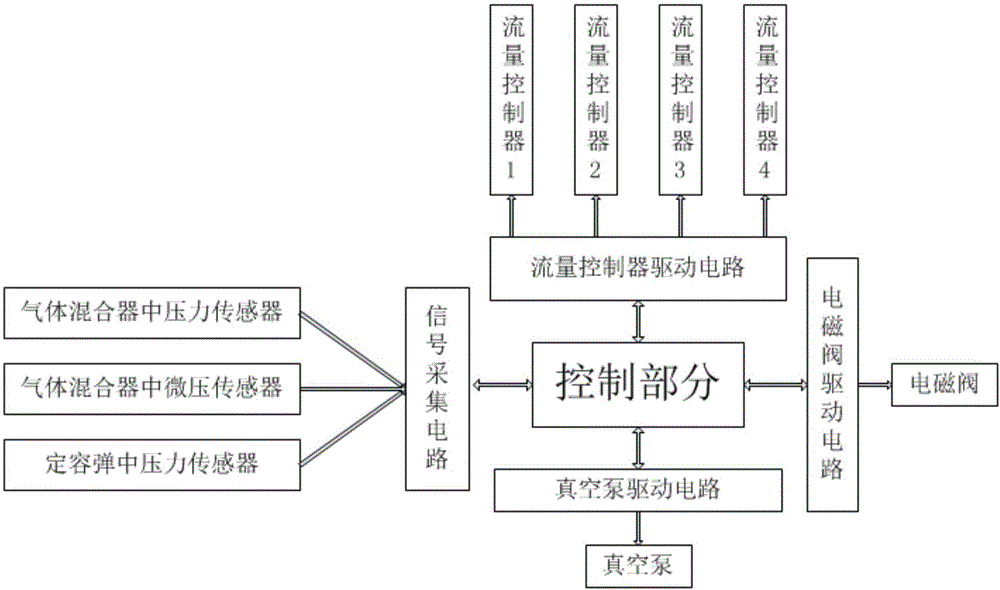
Constant volume bomb combustion gas mixing system and control method
InactiveCN106441909APrecise molar volume ratio controlMix completelyEngine testingGas cylinderEngineering
The present invention provides a constant volume bomb combustion gas mixing system and a control method. The system comprises gas cylinders of various types of gases, flow controllers, a gas mixer, a vacuum pump, a constant volume bomb, connecting pipes, a control part and a control circuit, wherein the connecting pipes are arranged between the gas cylinders, the flow controllers, the gas mixer, the vacuum pump and the constant volume bomb; the control circuit includes a vacuum pump driving circuit, a flow controller driving circuit, a signal acquisition circuit and a spark plug driving circuit; and the vacuum pump, the flow controllers, a pressure sensor which is arranged on the gas mixer, a micro pressure sensor, a pressure sensor which is arranged on the constant volume bomb, and a spark plug are all connected with the control part through the control circuit so as to be controlled, wherein the micro pressure sensor is arranged on the gas mixer, and the spark plug is arranged on the constant volume bomb. With the control method of the system adopted, precise molar volume ratio control on pre-combustion mixed gases can be realized, complete and controllable pre-combustion can be realized, a reliable environment can be provided for the search of the spray combustion characteristics of a diesel, experimental risk can be deceased, and high practicality can be realized.
Owner:HARBIN ENG UNIV
Preparing method of ferroferric oxide nanometer particles
ActiveCN104030363ASmall particle sizeSmall particlesMaterial nanotechnologyFerroso-ferric oxidesIonic liquidSodium hydroxide
The invention relates to a preparing method of ferroferric oxide nanometer particles with good dispersibility. The ferroferric oxide nanometer particles are prepared by steps of: adding ferric chloride and ferrous sulfate having the same molar as the ferric chloride into deionized water under the protection of an inert gas, so that the molar volume concentration of the ferrous sulfate containing ferrous iron and the molar volume concentration of the ferric chloride containing ferric iron are both maintained between 0.01-0.1 mol / L; adding a [BMIN]BF4 ionic liquid in place of a traditional surfactant; mixing uniformly; adding dropwise a sodium hydroxide solution adopted as a precipitator at 70 DEG C to precipitate to obtain a reaction product; washing alternatively with deionized water and absolute ethyl alcohol; centrifuging; and drying under vacuum. Raw materials used in the method are easily available, low in cost and short in preparation period. The obtained ferroferric oxide nanometer particles can be used as high-performance catalysts, magnetic materials and electrode materials.
Owner:瑞安市浙工大技术转移中心
Composition for the cold preparation of composite materials for adhesive bonding
InactiveUS20030062124A1Avoid sandingAvoidance of abrasion operationAdhesive processes with surface pretreatmentOrganic non-macromolecular adhesiveEtherKetone
The present invention relates to a liquid composition for the cold preparation of laminate composite materials so as to confer to them, without preliminary sanding or abrasion, a surface state favorable to adhesive bonding with polyurethane type adhesives comprising (a) at least one polar aprotic solvent (TPA); (b) at least one ether (TE) selected from the family of ethers, ether-esters, ether-ketones having: a molar volume less than 200 and preferably less than 160, a molecule devoid of hydroxyl function, and (c) at least one activator (TA) comprising at least one reactive nitrogenous function of the -NH2 and / or -NH- type of molar volume less than 100.
Owner:ATO FINDLEY
Oxygen removal systems during fuel cell shutdown
A purging system for removing oxygen from a fuel cell system during a shutdown period for the fuel cell system. The purging system includes a separator having an inlet and an outlet; a first exhaust line for communicating a first exhaust gas stream from an outlet of the fuel cell system to the separator inlet during the shutdown period of the fuel cell system; and a second exhaust line for communicating a second exhaust gas stream to an inlet of the fuel cell system for delivering the second exhaust gas stream to the fuel cell system during the shutdown period. The separator removes oxygen from the first exhaust gas stream such that the first stream nitrogen molar volume is lower than the second steam nitrogen molar volume and the first stream oxygen molar volume is higher than the second stream oxygen molar volume.
Owner:FORD MOTOR CO
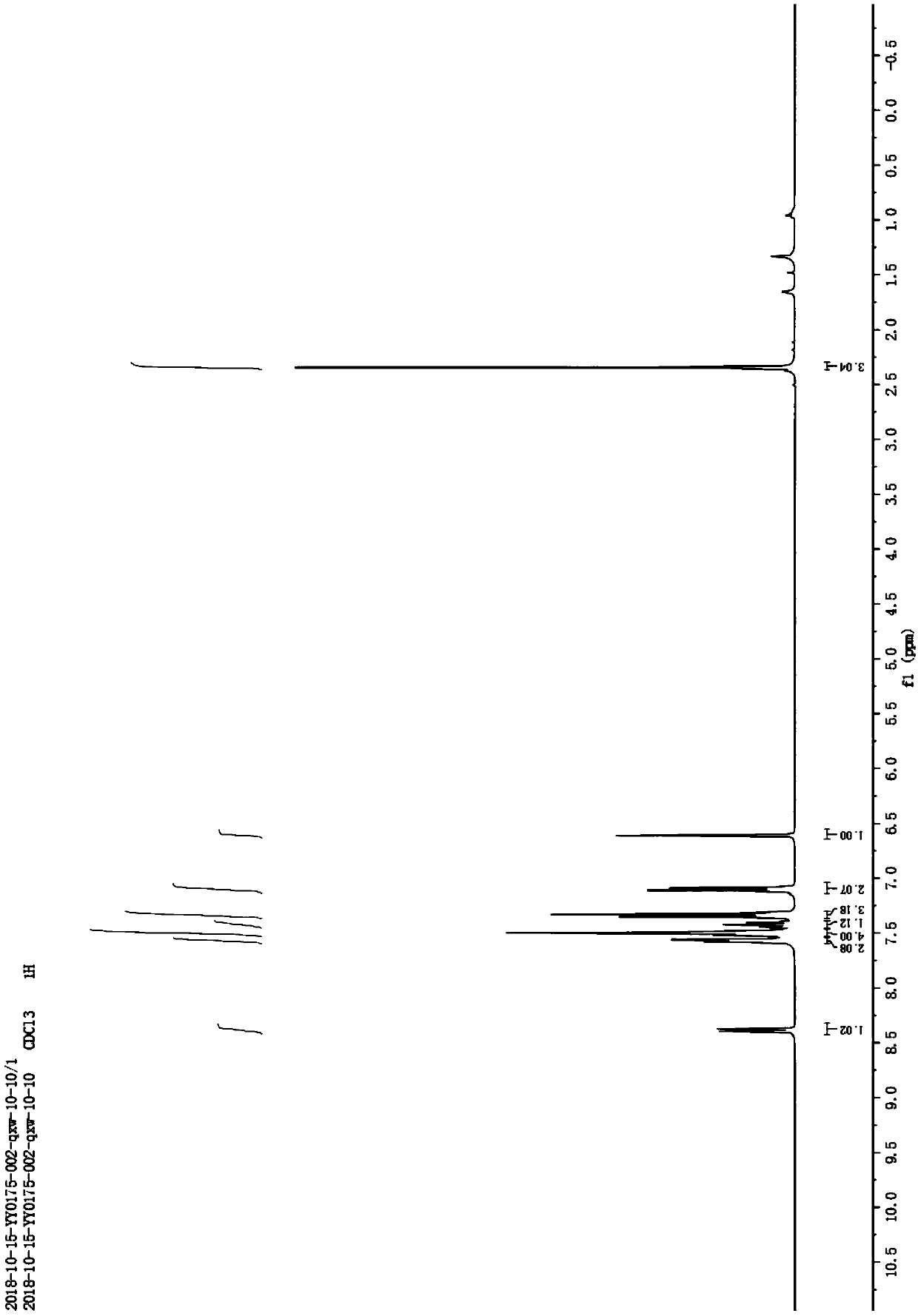
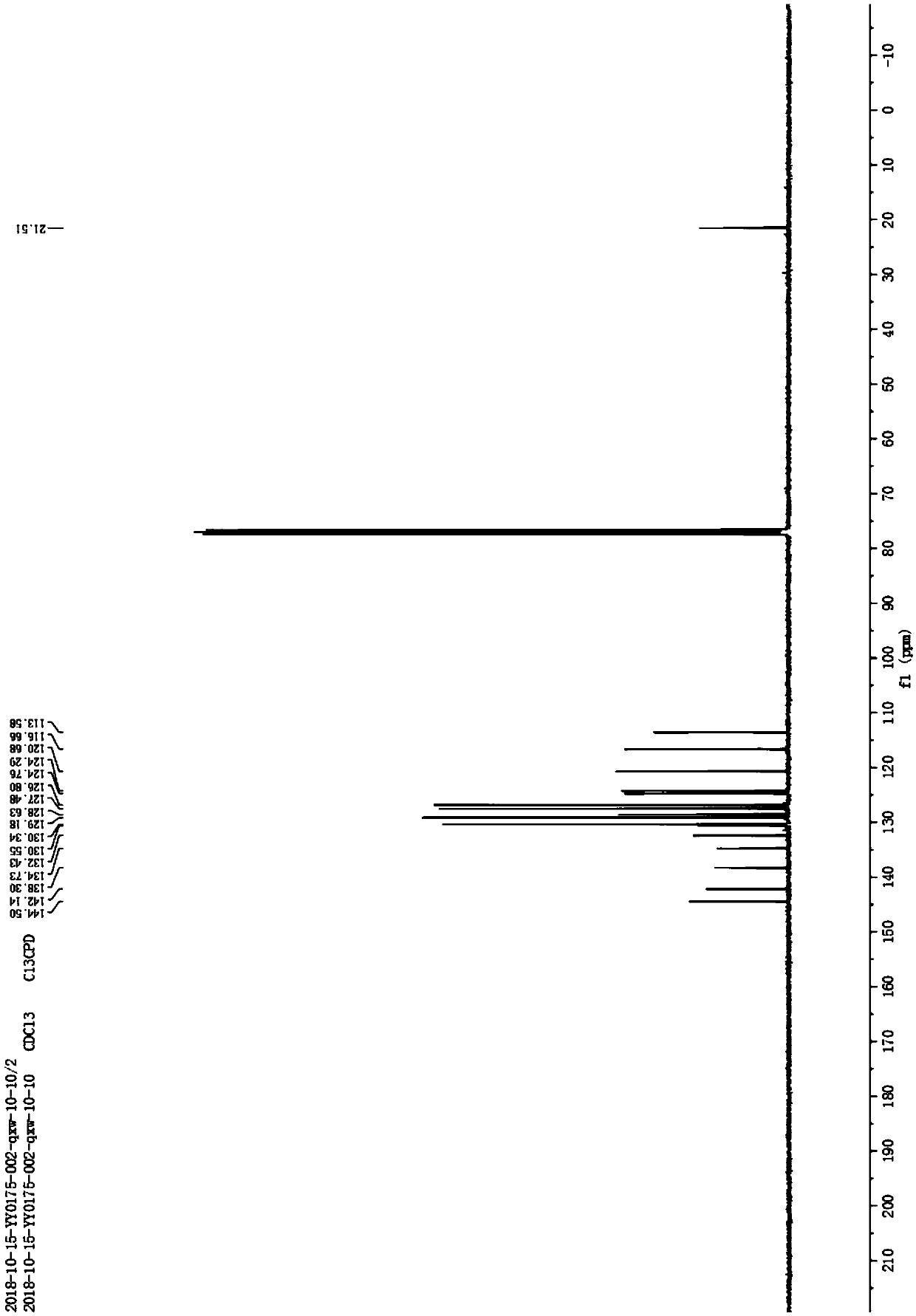
Catalytic synthesis method of 2-substituted benzofuran compound
The invention discloses a catalytic synthesis method of a 2-substituted benzofuran compound. The catalytic synthesis method includes the steps of dissolving 2-alkynyl substituted phenol in acetonitrile under normal temperature and normal pressure to obtain an acetonitrile solution of the 2-alkynyl substituted phenol, wherein the molar volume ratio of the 2-alkynyl substituted phenol to the acetonitrile is 1:1mmol / mL; adding cuprous chloride and cesium carbonate to the acetonitrile solution, wherein the molar ratio of each of the cuprous chloride and the cesium carbonate is 5% of that of the 2-alkynyl substituted phenol; stirring the solution at room temperature for 6 hours to generate the 2-substituted benzofuran compound after reaction. The catalytic synthesis method of the 2-substitutedbenzofuran compound has low cost and high yield; the cost of a catalyst required for preparing 1g of the 2-substituted benzofuran compound is reduced by two orders of magnitude as compared with that of existing synthesis methods in which iridium catalysts are used, and the yield is increased to 88.5 to 95.8%.
Owner:ZHEJIANG WANLI UNIV
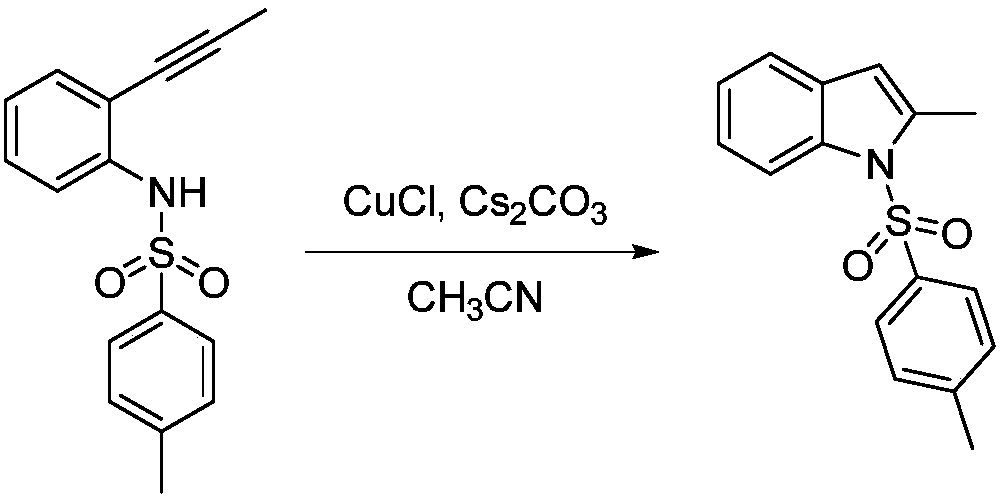
Catalytic synthesis method of N-p-toluenesulfonyl-2-substituted indole compound
The invention discloses an N-p Toluenesulfonyl-2-the catalytic synthesis method of substituted indole compound, which includes the following steps: under the condition of normal temp and normal pressure, N-p -Toluenesulfonyl-2-alkynyl-substituted aniline is dissolved in acetonitrile, and the molar volume ratio of N-p -Toluenesulfonyl-2-alkynyl-substituted aniline to acetonitrile is 1.1 mmol / mL togive acetonitrile solution of N-P-Toluenesulfonyl-2-alkynyl-substituted aniline; adding cuprous chloride and cesium carbonate into acetonitrile solution, wherein the molar amounts of the cuprous chloride and the cesium carbonate are respectively 5% of molar amount of N-p -Toluenesulfonyl-2-alkynyl-substituted aniline; thereafter, the mixture is stirred at room temperature for 6 hours, and the reaction is carried out to give N-P-Toluenesulfonyl-2-substituted indole compounds. The synthesis method has low cost and high yield, and the cost of the catalyst for preparing 1g of N-P-Toluenesulfonyl-2-substituted indole compound is reduced by three orders of magnitude compared with the prior synthesis method taking the rhodium catalyst as the catalyst, and the yield is improved to 89.1-97.0%.
Owner:ZHEJIANG WANLI UNIV
Multicolor fluorescent material regulated through crystal forms and preparation method and application thereof
InactiveCN105238396AMeet diverse application needsLow costLuminescent compositionsSemiconductor devicesRare earth ionsDisplay device
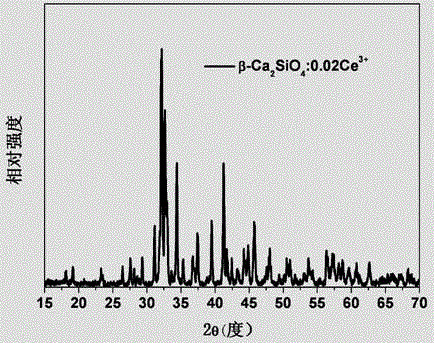
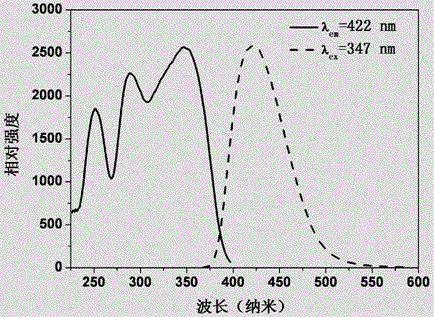
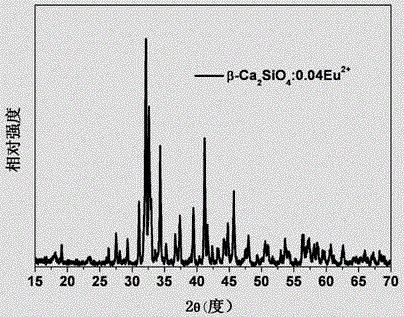
The invention provides a multicolor fluorescent material regulated through crystal forms. The general chemical formula of the material is Ca2SiO4:xRe,yM, wherein Re is a rare earth ion Ce<3+> or Eu<2+>, M is a doping agent for regulating the crystal forms, the additive quantity of Re is 0.001<=x<=0.4 by molar volume, and the additive quantity of M is 0.00<=y<=1.0 by molar volume; the Ca2SiO4 fluorescent material with the light-emitting color adjustable and five kinds of the crystal forms of gamma-, beta-, alpha'L-, alpha'H- and alpha- is prepared and synthesized by doping the crystal form regulating agent M, and the fluorescent material can achieve color-adjustable emission of blue light, green light, yellow light and red light in the whole visible light range under excitation of near ultraviolet or blue light. The multicolor fluorescent material regulated through the crystal forms has the advantages that the different crystal forms can be obtained only by doping the crystal form regulating agent, so that the fluorescent material with the different light-emitting colors is prepared; the preparation and synthesis technology is simple, the cost is low, and the potential application prospect on LED illumination and display devices are achieved.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Preparation method of liquid calcium citrate malate, liquid calcium citrate malate oral solution and preparation methods thereof
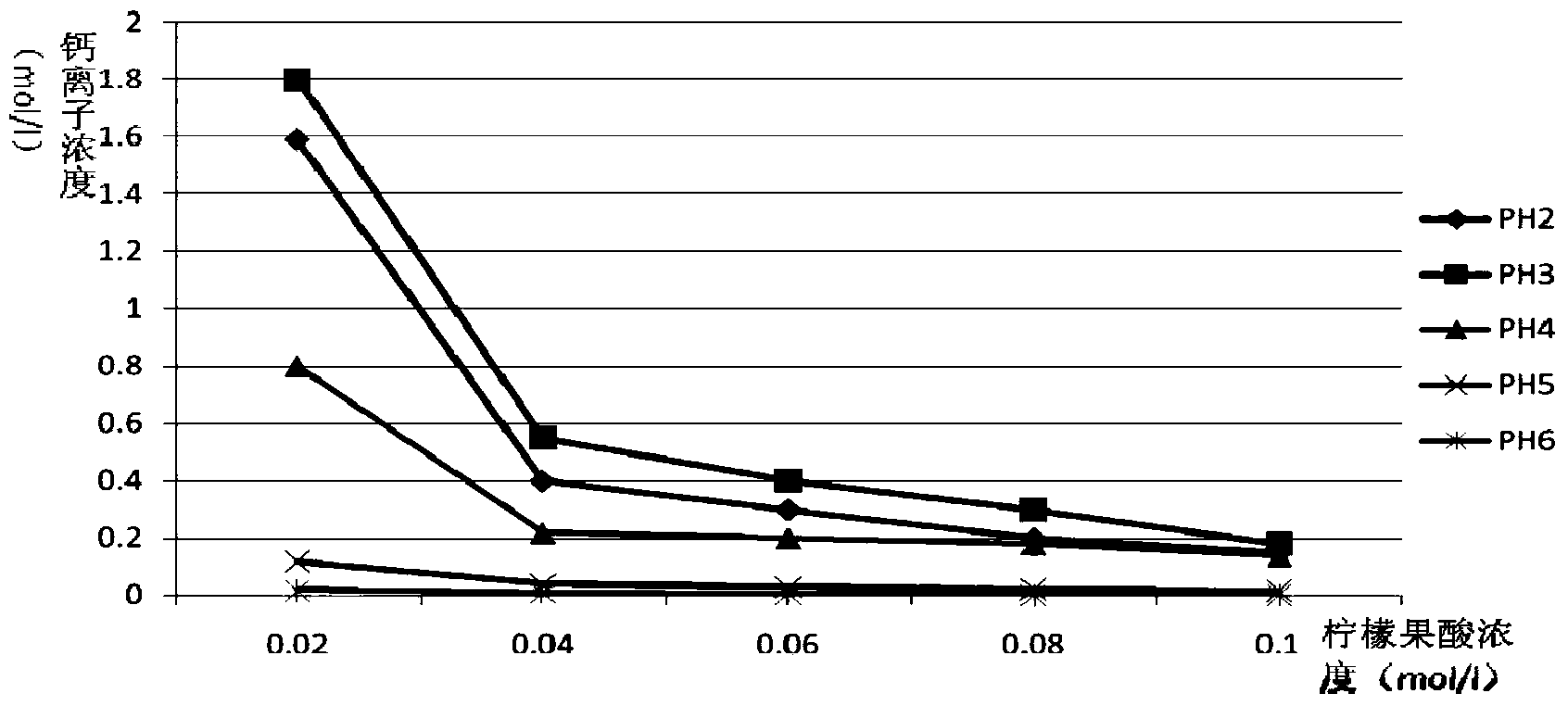
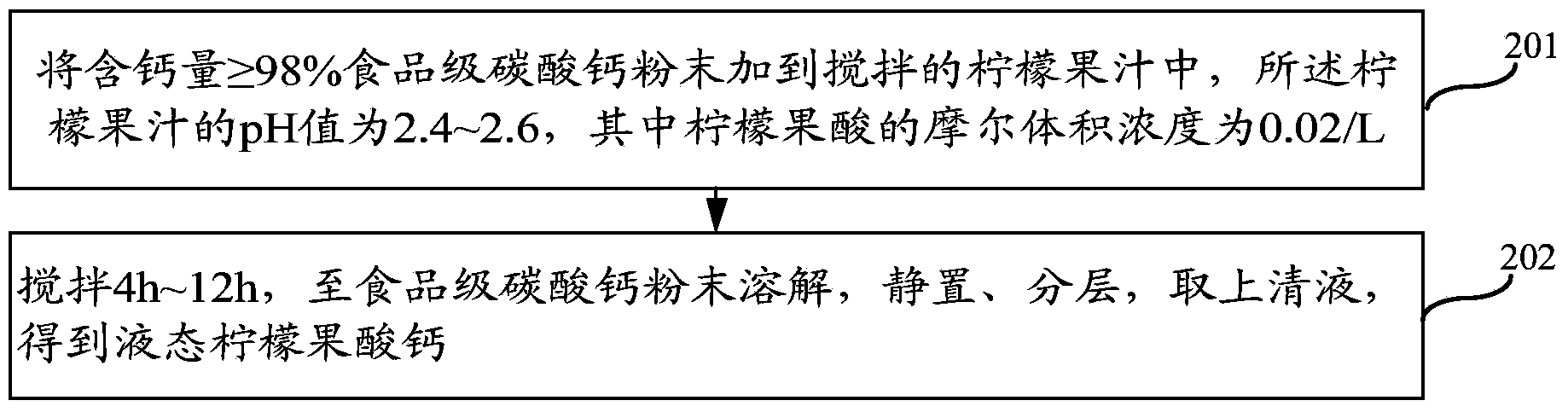
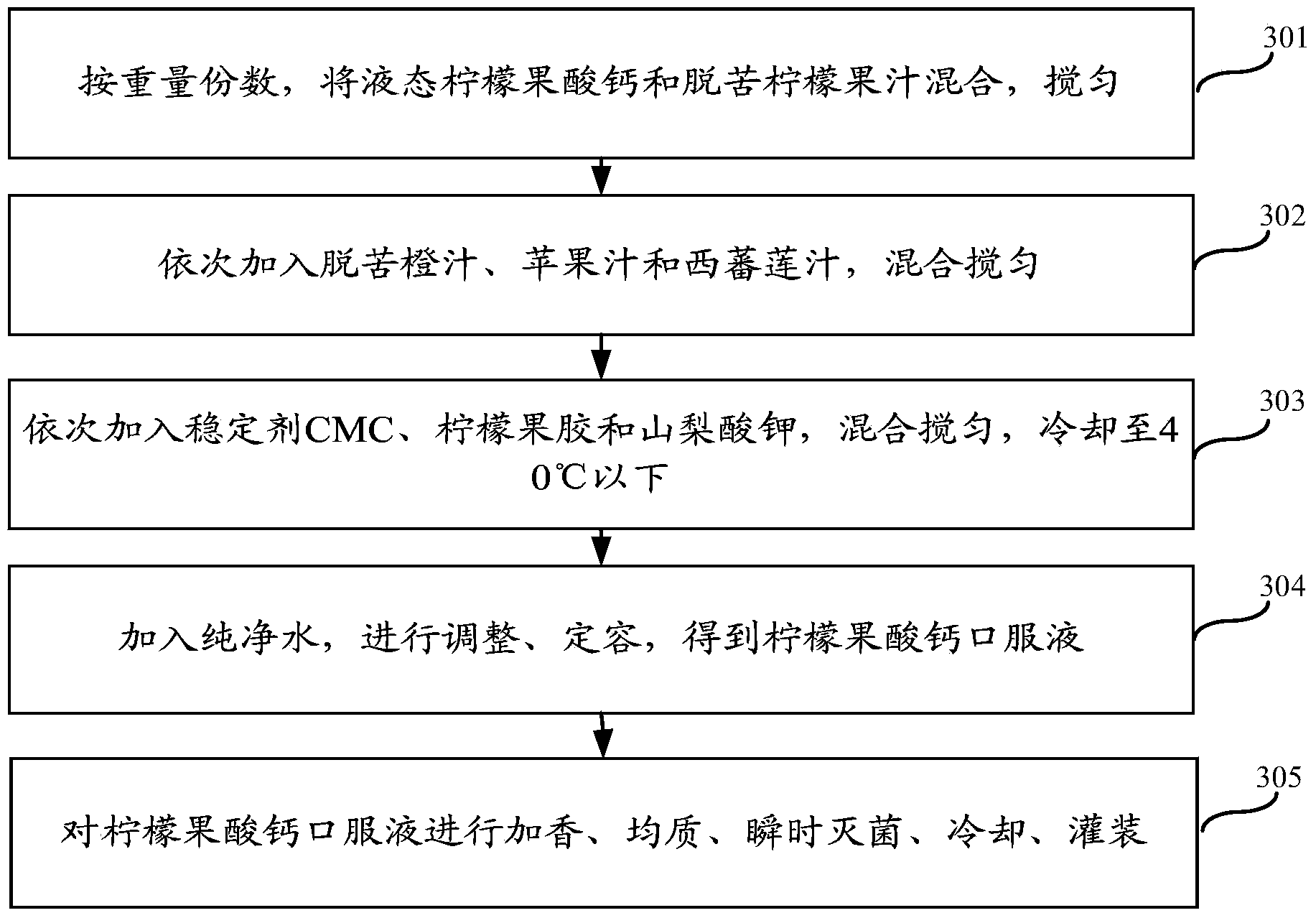
ActiveCN103859421AImprove solubilityPromote absorptionFood ingredientsFood preparationFruit juiceVitamin C
The invention relates to the field of health products, in particular to a preparation method of liquid calcium citrate malate; in addition, the invention also relates to a liquid calcium citrate malate oral solution and a preparation method thereof. The preparation method of liquid calcium citrate malate comprises the following steps: adding food-grade calcium carbonate powder with the calcium content of greater or larger than 98% into a stirred lemon juice with a pH value of 2.4-2.6 and a molar volume concentration of 0.02mol / L; and stirring for 4-12h until the food-grade calcium carbonate powder is thoroughly dissolved, performing standing and layering, getting supernatant to obtain the liquid calcium citrate malate. The liquid calcium citrate malate oral solution prepared by adopting the preparation method provided by the invention is rich in calcium citrate malate, citrate malate, citrate, vitamin C and many nutritional ingredients which are needed by a human body, such as amino acid and rare elements; the liquid calcium citrate malate oral solution is easy to take and is good in taste.
Owner:SICHUAN HUATONG LEMON
Polymer-containing organo-metal catalysts
InactiveUS20030162654A1Organic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationMolar volumeOrganometallic catalysis
The invention relates to polymer-containing catalysts (e.g., polymer-containing organo-metal catalysts) that may be useful in various applications (e.g., polymerization of macrocyclic oligoesters). Advantages of these catalysts include the improved ability to prepare, store, and handle the catalyst in open air; higher molar volume and molecular weight; and the ability to produce a blend of reactants and a catalyst (e.g., a macrocyclic oligoester and a polymerization catalyst) for subsequent one-step reaction (e.g., polymerization of macrocyclic oligoesters).
Owner:CYCLICS CORP
Method for preparing basic magnesium carbonate by using combinative alkali system high-salt heavy-ash mother solution
InactiveCN104445304ASimple production processShorten the production cycleMagnesium carbonatesMagmaChloride
The invention discloses a method for preparing basic magnesium carbonate by using a combinative alkali system high-salt heavy-ash mother solution. The method comprises the following steps: (1) dissolving industrial magnesium chloride into a solution with the concentration of 1-1.45mol / L, heating to 80-90 DEG C, and filtering to remove insoluble substances; (2) heating a high-salt heavy-ash mother solution to 50-60 DEG C, keeping the temperature constant, and then filtering to remove calcium ion impurities; (3) adding the filtered high-salt heavy-ash mother solution into the filtered industrial magnesium chloride solution while stirring for fully reacting, and then keeping the temperature of the solution after reaction for 2-3 hours, wherein the molar volume ratio of the added amounts of the high-salt heavy-ash mother solution and the industrial magnesium chloride solution is 1.2-1.5; and (4) filtering a magma solution, washing by using deionized water at the same time, and drying at 120-130 DEG C to obtain a basic magnesium carbonate product. The magnesium oxide content of the basic magnesium carbonate prepared by the method disclosed by the invention is about 41% and can reach an industrial standard.
Owner:TIANJIN BOHUA YONGLI CHEM IND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com