Multicolor fluorescent material regulated through crystal forms and preparation method and application thereof
A technology for controlling fluorescent materials and crystal forms, which can be used in luminescent materials, chemical instruments and methods, electrical components, etc., and can solve the problems of easy deliquescence, poor chemical stability of sulfide fluorescent materials, and difficulty in obtaining raw materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
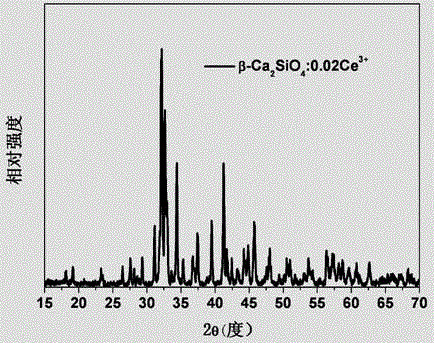
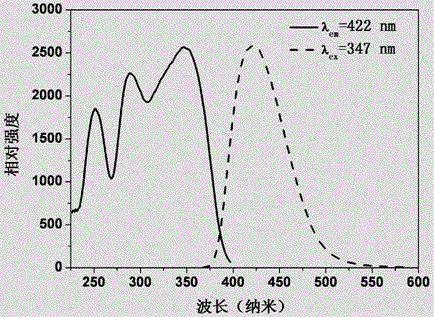
[0035] A method for preparing a multicolor fluorescent material controlled by crystal form, the chemical formula of the multicolor fluorescent material is Ca 2 SiO 4 : 0.02Ce 3+ , the preparation steps are as follows:
[0036] According to the chemical formula of the material Ca 2 SiO 4 : 0.02Ce 3+ Accurately Weigh CaCO 3 , SiO 2 and CeO 2 Raw material, Ce 3+ Ions replace Ca 2 SiO 4 Ca in 2+ Ion measurement: put the weighed raw materials into an agate mortar, add 50% alcohol as a grinding medium, mix and dry them evenly, and put them into a corundum crucible. 2 The volume percentage is 10% N 2 +H 2 The mixed gas is a reducing atmosphere, calcined in a horizontal tube furnace at 1450°C for 12 hours, taken out for grinding, put into the tube furnace again and calcined at 1400°C for 6 hours in the same reducing atmosphere, and naturally cooled to room temperature to obtain fluorescence Material. The XRD diffraction pattern of the fluorescent material that present e...
Embodiment 2
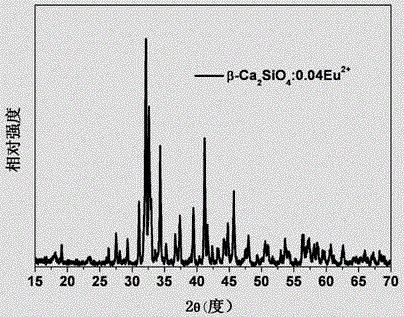
[0038] A method for preparing a multicolor fluorescent material controlled by crystal form, the chemical formula of the multicolor fluorescent material is Ca 2 SiO 4 : 0.04Eu 2+ , the preparation steps are as follows:
[0039] According to the chemical formula of the material Ca 2 SiO 4 : 0.04Eu 2+ Accurately Weigh CaCO 3 , SiO 2 and Eu 2 o 3 Raw materials, Eu 2+ Ions replace Ca 2 SiO 4 Ca in 2+ Ion measurement: put the weighed raw materials into an agate mortar, add 50% alcohol as a grinding medium, mix and dry them evenly, and put them into a corundum crucible. 2 The volume percentage is 10% N 2 +H 2 The mixed gas is a reducing atmosphere, calcined in a horizontal tube furnace at 1450°C for 12 hours, taken out for grinding, put into the tube furnace again and calcined at 1400°C for 6 hours in the same reducing atmosphere, and naturally cooled to room temperature to obtain fluorescence Material. The XRD diffraction pattern of the fluorescent material that pres...
Embodiment 3
[0041] A method for preparing a multicolor fluorescent material controlled by crystal form, the chemical formula of the multicolor fluorescent material is Ca 2 SiO 4 : 0.02Ce 3+ ,0.04Al 3+ , the preparation steps are as follows:
[0042] According to the chemical formula of the material Ca 2 SiO 4 : 0.02Ce 3+ ,0.04Al 3+ Accurately Weigh CaCO 3 , SiO 2 , CeO 2 and Al 2 o 3 Raw material, Ce 3+ and Al 3+ ions respectively replace Ca 2 SiO 4 Ca in 2+ and Si 4+ Ion measurement: put the weighed raw materials into an agate mortar, add 50% alcohol as a grinding medium, mix and dry them evenly, and put them into a corundum crucible. 2 The volume percentage is 10% N 2 +H 2 The mixed gas is a reducing atmosphere, calcined in a horizontal tube furnace at 1450°C for 12 hours, taken out for grinding, put into the tube furnace again and calcined at 1400°C for 6 hours in the same reducing atmosphere, and naturally cooled to room temperature to obtain fluorescence Material....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com