Catalytic synthesis method of N-p-toluenesulfonyl-2-substituted indole compound
The technology of a p-toluenesulfonyl group and a synthesis method, which is applied in the field of compound synthesis, can solve the problems of high price, high preparation cost, high temperature and the like, and achieves the effects of low cost, improved yield and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
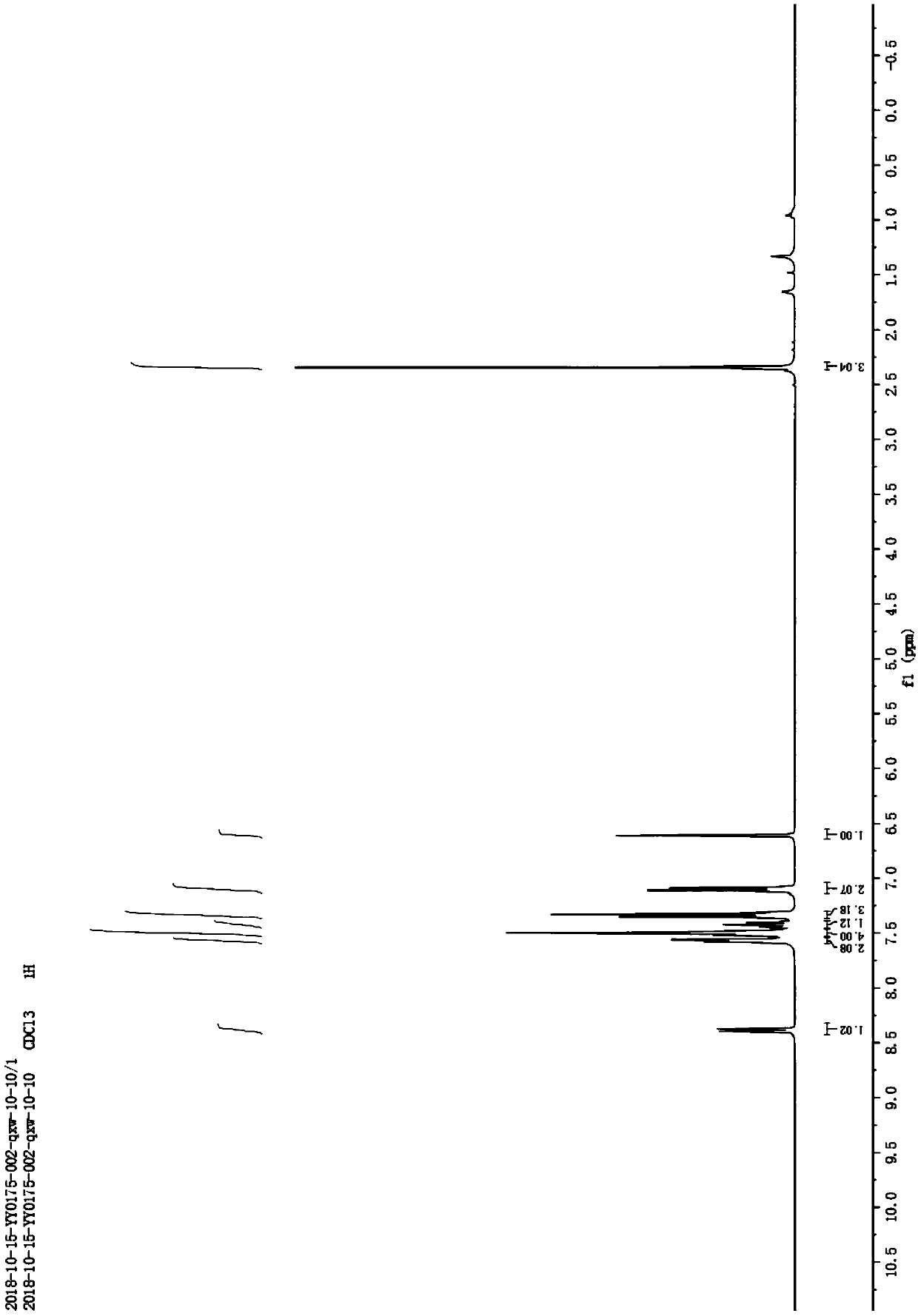
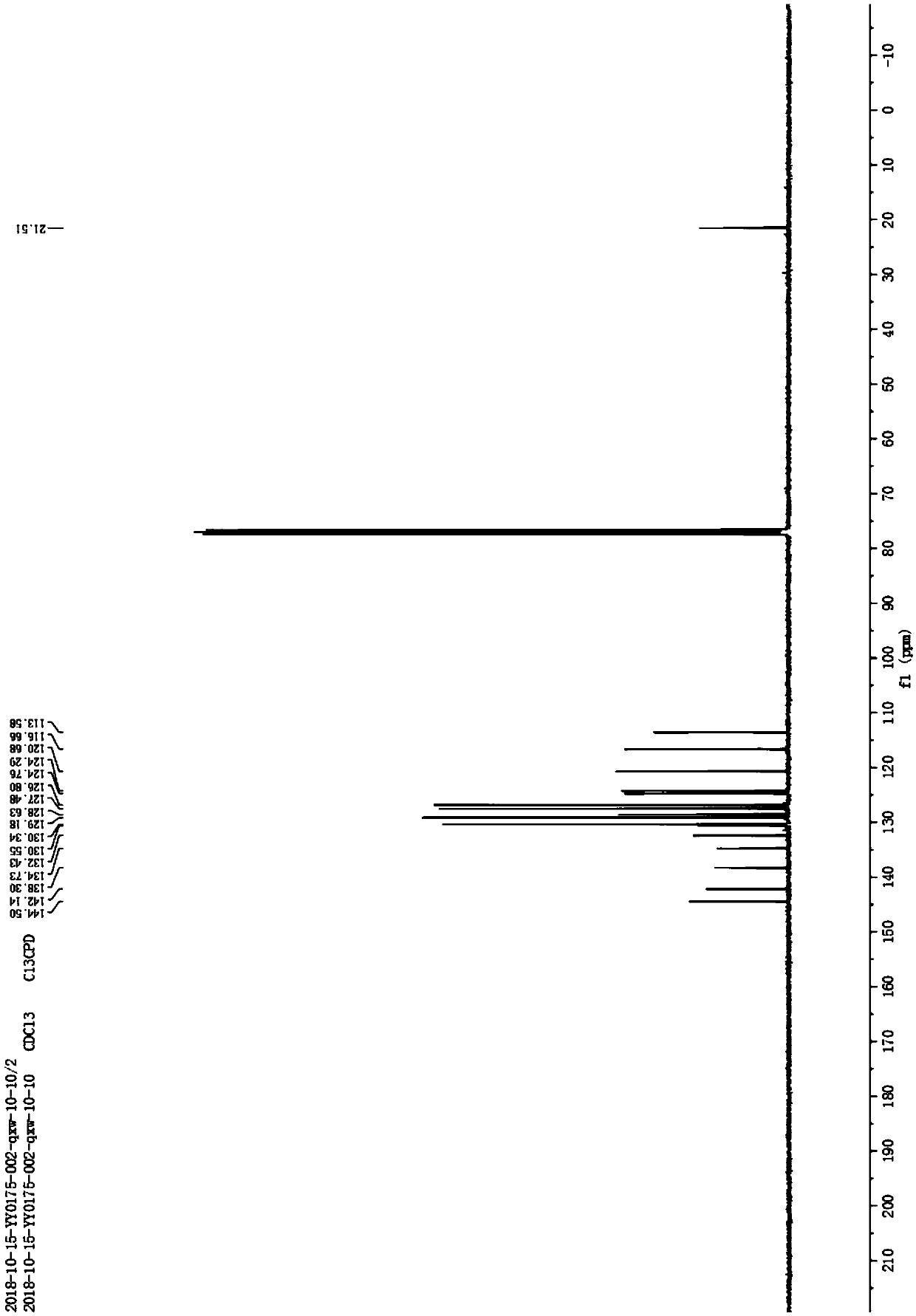
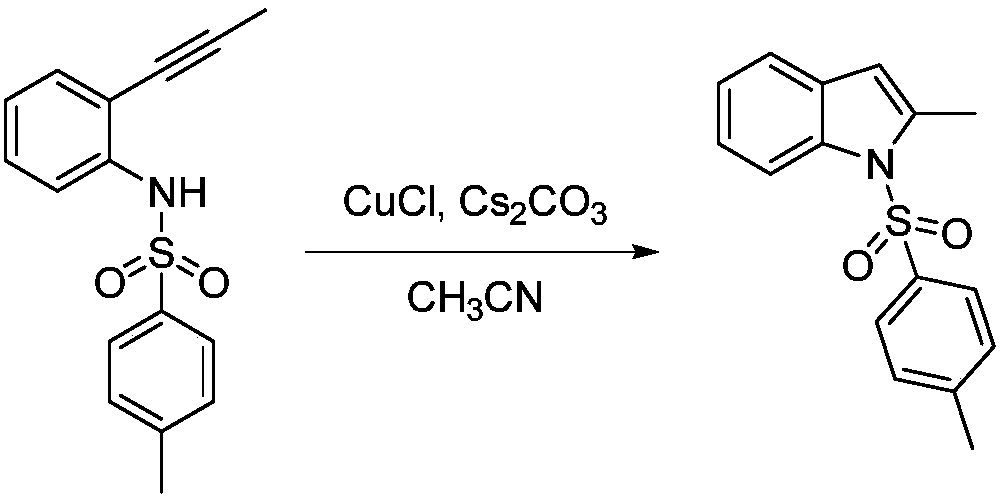
[0010] Example 1: Under normal temperature and pressure, 10mmol N-p-toluenesulfonyl-2-(2-methyl)ethynylaniline was dissolved in 10mL of acetonitrile to obtain N-p-toluenesulfonyl-2-(2-methyl ) the acetonitrile solution of ethynylaniline; then add 0.5mmol cuprous chloride and 0.5mmol cesium carbonate to the acetonitrile solution; after that, stir at room temperature with a stirring rate of 600r / min for 6 hours, the reaction generates N-p-toluenesulfonyl-2 -Methylindole, N-p-toluenesulfonyl-2-methylindole 2.6g was obtained through silica gel column chromatography, and its yield was 91.1%. Taking N-toluenesulfonyl-2-(2-methyl)ethynylaniline as the reaction formula of raw material synthesis N-toluenesulfonyl-2-methylindole in embodiment 1 is:
[0011]
[0012] After accounting, in Example 1, the cost of the required catalyst for every 1g of N-toluenesulfonyl-2-methylindole prepared is only 0.2 yuan, which is 3 yuan lower than the existing synthetic method using a rhodium cataly...
Embodiment 2
[0013] Example 2: Under normal temperature and pressure, 20mmol N-p-toluenesulfonyl-2-(2-ethyl)ethynylaniline was dissolved in 20mL of acetonitrile to obtain N-p-toluenesulfonyl-2-(2-ethyl ) the acetonitrile solution of ethynylaniline; then add 1.0mmol cuprous chloride and 1.0mmol cesium carbonate to the acetonitrile solution; after that, stir at room temperature with a stirring rate of 700r / min for 6 hours, the reaction generates N-p-toluenesulfonyl-2 - Ethyl indole, 5.6 g of N-p-toluenesulfonyl-2-ethyl indole was obtained by silica gel column chromatography, and the yield was 93.5%. Taking N-p-toluenesulfonyl-2-(2-ethyl) ethynyl aniline as the reaction formula of raw material synthesis N-p-toluenesulfonyl-2-ethylindole in embodiment 2 is:
[0014]
[0015] After accounting, in Example 2, the cost of the required catalyst for every 1g of N-toluenesulfonyl-2-ethylindole is only 0.19 yuan, which is 3 yuan lower than the existing synthetic method using rhodium catalyst as cat...
Embodiment 3
[0016] Example 3: Under normal temperature and pressure, 40mmol N-p-toluenesulfonyl-2-(2-butyl)ethynylaniline was dissolved in 40mL of acetonitrile to obtain N-p-toluenesulfonyl-2-(2-butyl ) the acetonitrile solution of ethynylaniline; then add 2.0mmol cuprous chloride and 2.0mmol cesium carbonate to the acetonitrile solution; after that, stir at room temperature with a stirring rate of 500r / min for 6 hours, and the reaction generates N-p-toluenesulfonyl-2 -Butylindole, N-p-toluenesulfonyl-2-butylindole 12.4g was obtained through silica gel column chromatography, and its yield was 94.7%. Taking N-p-toluenesulfonyl-2-(2-butyl)ethynylaniline as the reaction formula of raw material synthesis N-p-toluenesulfonyl-2-butylindole in embodiment 3 is:
[0017]
[0018] After accounting, in Example 3, the cost of the required catalyst for every 1g of N-toluenesulfonyl-2-butylindole prepared is only 0.17 yuan, which is 3 yuan lower than the existing synthetic method using a rhodium cat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com