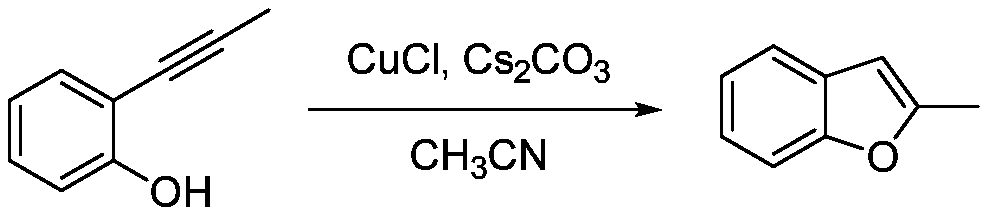Catalytic synthesis method of 2-substituted benzofuran compound
A technology of benzofuran and synthesis method, which is applied in the field of catalytic synthesis of 2-substituted benzofuran compounds, can solve the problems of high preparation cost and high price, and achieve the effects of low cost, increased yield, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
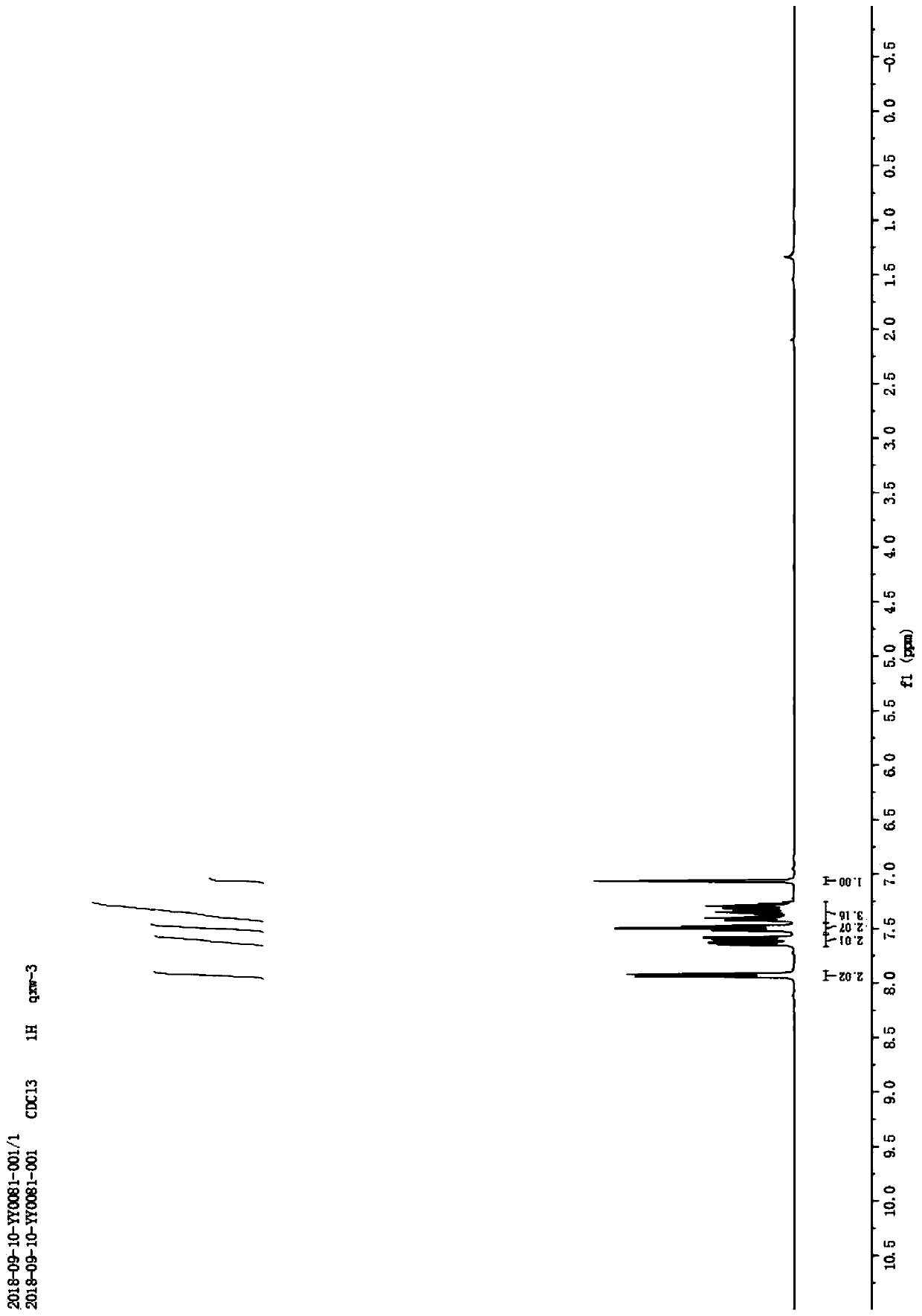
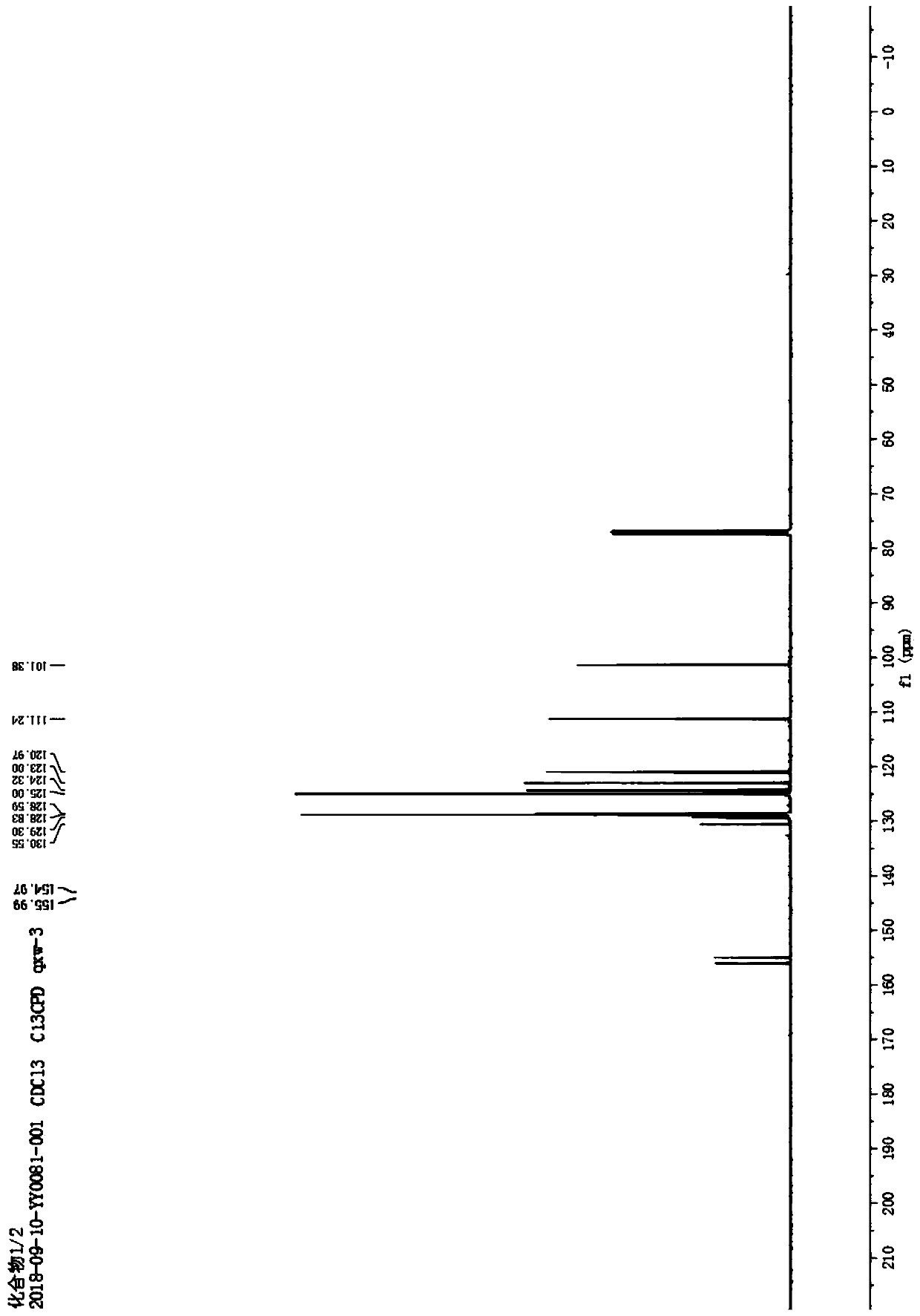
[0010] Example 1: Under normal temperature and pressure, 10mmol of 2-(2-methyl)ethynylphenol was dissolved in 10mL of acetonitrile to obtain an acetonitrile solution of 2-(2-methyl)ethynylphenol; 0.5mmol cuprous chloride and 0.5mmol cesium carbonate; after that, stir at room temperature for 6 hours at a stirring rate of 600r / min, the reaction generates 2-methylbenzofuran, and 2-methylbenzofuran 1.2 is obtained by silica gel column chromatography g, its yield is 90.8%. Taking 2-(2-methyl)ethynylphenol as the reaction formula of raw material synthesis 2-methylbenzofuran in embodiment 1 is:
[0011]
[0012] According to calculations, in Example 1, the cost of the catalyst required to prepare 1 g of 2-methylbenzofuran is only 0.44 yuan, which is 2 orders of magnitude lower than the existing synthesis method using iridium catalyst as the catalyst.
Embodiment 2
[0013] Example 2: Under normal temperature and pressure, 20mmol 2-(2-ethyl)ethynylphenol was dissolved in 20mL acetonitrile to obtain an acetonitrile solution of 2-(2-ethyl)ethynylphenol; 1.0mmol cuprous chloride and 1.0mmol cesium carbonate; after that, stir at room temperature for 6 hours at a stirring rate of 500r / min, the reaction generates 2-ethylbenzofuran, and 2-ethylbenzofuran 2.8 is obtained by silica gel column chromatography g, its yield is 95.8%. Taking 2-(2-ethyl)ethynylphenol as the reaction formula of raw material synthesis 2-ethylbenzofuran in embodiment 2 is:
[0014]
[0015] According to calculations, in Example 2, the cost of the catalyst required to prepare 1 g of 2-ethylbenzofuran is only 0.37 yuan, which is 2 orders of magnitude lower than the existing synthesis method using iridium catalyst as the catalyst.
Embodiment 3
[0016] Example 3: Under normal temperature and pressure, 40mmol 2-(2-butyl)ethynylphenol was dissolved in 40mL of acetonitrile to obtain an acetonitrile solution of 2-(2-butyl)ethynylphenol; 2.0mmol cuprous chloride and 2.0mmol cesium carbonate; after that, stir at room temperature at a stirring rate of 800r / min for 6 hours, the reaction generates 2-butylbenzofuran, and 2-butylbenzofuran 6.4 is obtained by silica gel column chromatography g, its yield is 91.8%. Taking 2-(2-butyl) ethynylphenol as the reaction formula of raw material synthesis 2-butylbenzofuran in embodiment 3 is:
[0017]
[0018] According to calculations, in Example 3, the cost of the catalyst required to prepare 1 g of 2-butylbenzofuran is only 0.33 yuan, which is 2 orders of magnitude lower than the existing synthesis method using iridium catalyst as the catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com