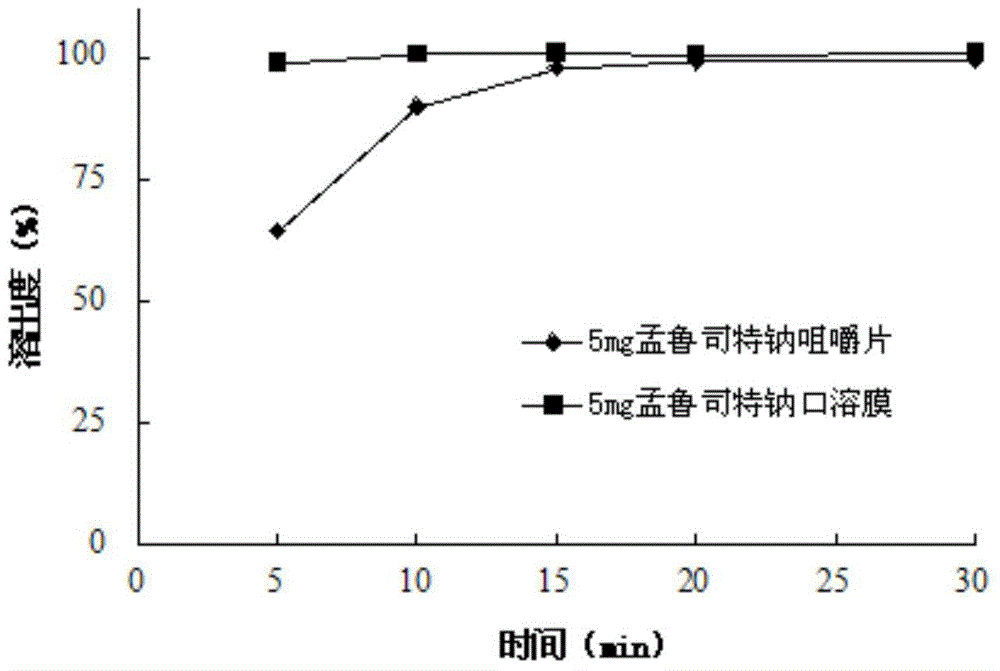
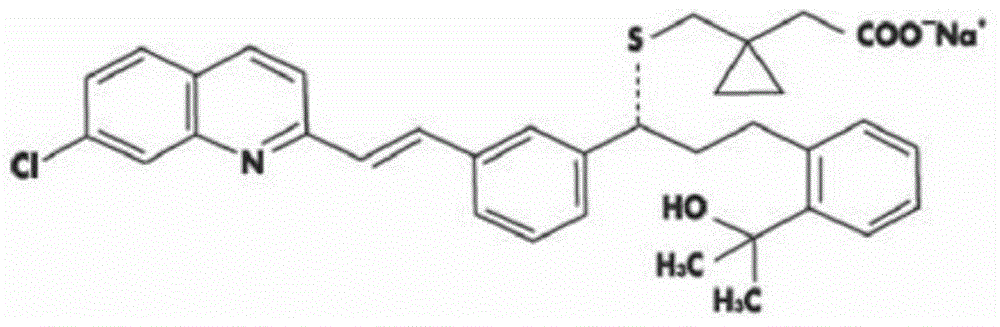
Stable Montelukast oral film preparation
An oral film and stabilizer technology, applied in the field of medicine, can solve the problems of high safety risk, inducing cancer, loss of activity, etc., and achieve the effect of avoiding Tibetan medicine, good taste and bright color
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
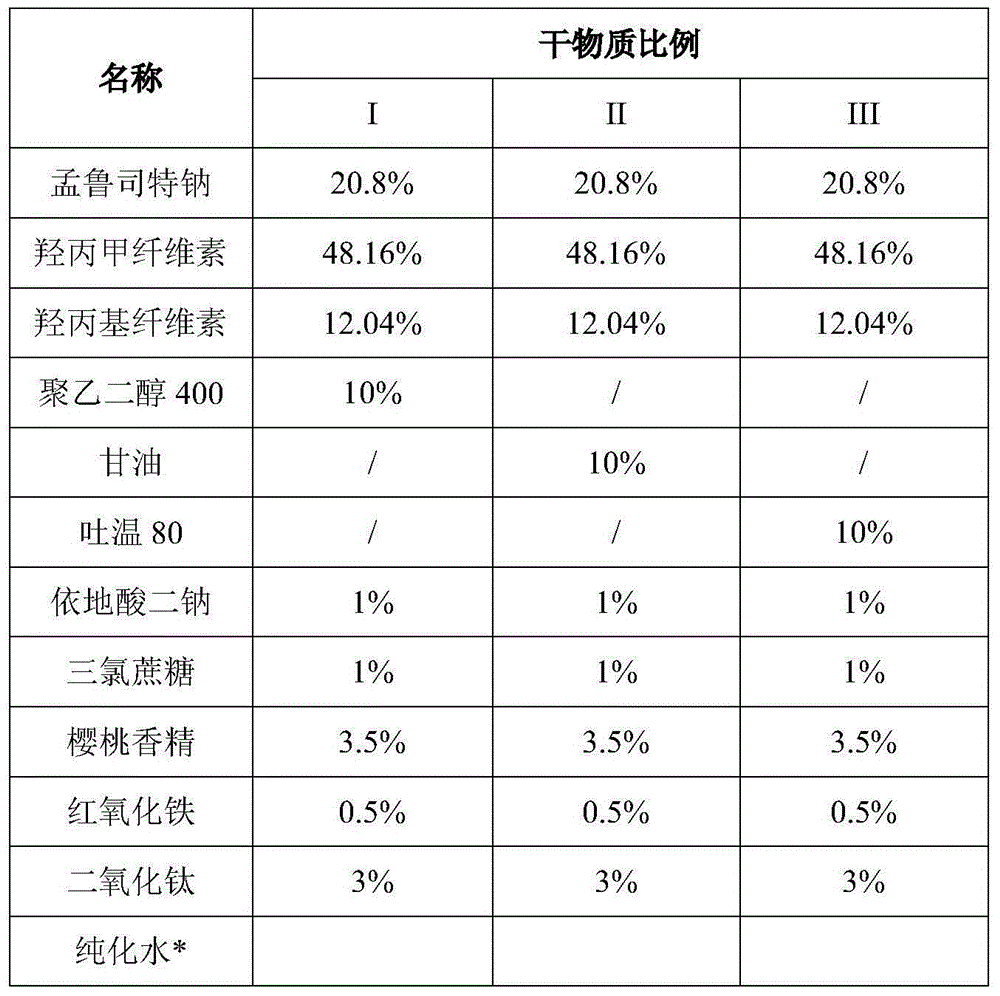
[0035] The influence of embodiment 1 disodium edetate on product stability
[0036]
[0037] *Used in prescription but removed during processing.
[0038] Preparation process: Dissolve or disperse all components except montelukast sodium and film-forming material in water under stirring under dark conditions, add montelukast sodium and stir to dissolve to obtain a drug-containing solution; add film-forming material , stirred until completely dissolved to obtain the drug-containing glue; stirred and defoamed under vacuum conditions, evenly coated the drug-containing glue on the polyester tape with a scraper, heated and dried, and cut into certain sizes to obtain pink Montelukast sodium orally dissolving film.
Embodiment 2
[0048] Embodiment 2 The impact of different dosages of edetate disodium on product stability
[0049]
[0050]
[0051] *Used in prescription but removed during processing.
[0052] Preparation process: Dissolve or disperse all components except montelukast sodium and film-forming material in water under stirring under dark conditions, add montelukast sodium and stir to dissolve to obtain a drug-containing solution; add film-forming material , stirred until completely dissolved to obtain the drug-containing glue; stirred and defoamed under vacuum conditions, evenly coated the drug-containing glue on the polyester tape with a scraper, heated and dried, and cut into certain sizes to obtain pink Montelukast sodium orally dissolving film.
Embodiment 3
[0065] Embodiment 3 The impact of different types of stabilizers on product stability
[0066]
[0067] *Used in prescription but removed during processing.
[0068] Preparation process: Dissolve or disperse all components except montelukast sodium and film-forming material in water under stirring under dark conditions, add montelukast sodium and stir to dissolve to obtain a drug-containing solution; add film-forming material , stirred until completely dissolved to obtain the drug-containing glue; stirred and defoamed under vacuum conditions, evenly coated the drug-containing glue on the polyester tape with a scraper, heated and dried, and cut into certain sizes to obtain pink Montelukast sodium orally dissolving film.
[0069] The sample of Example 3 was placed under the conditions of 40° C. and 75% relative humidity for 30 days, and samples were taken at 0, 15, and 30 days for determination according to the method of Test Example 1. The result is as follows:
[0070] T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| breaking force | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com