Combination of cyclosporine A and alkylation serum albumin and preparation method thereof
A serum albumin and composition technology, which is applied in the direction of non-active ingredients of polymer compounds, drug combinations, medical preparations of non-active ingredients, etc., can solve problems such as hypersensitivity, liver and kidney toxicity, and achieve low irritation, The effect of high drug loading and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Cyclosporin A Octyl Human Serum Albumin Nanoparticles
[0026] Take 90mg of octyl-modified human serum albumin derivative, dissolve it in 15mL double-distilled water, stir at room temperature for 0.5h to fully swell; ; Probe ultrasound (200w, 30min); dialysis bag (MWCO=12,000~14,000) single-distilled water dialysis for 8h, the resulting nano-solution passed through a 0.45μm microporous membrane; add 1% (w / v) mannitol to the filtrate, mix well , aliquoted and freeze-dried.
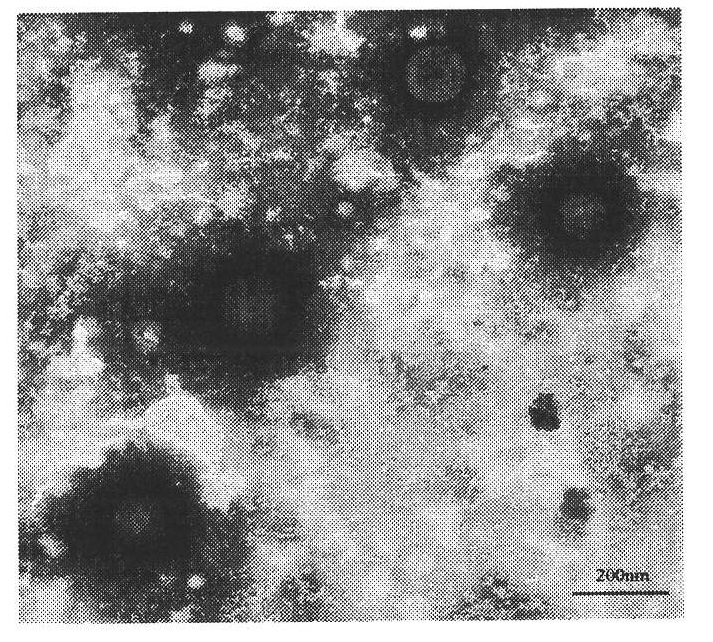
[0027] The test results showed that the cyclosporin A octyl human serum albumin nanoparticles had a drug loading capacity of 31.14%, an encapsulation efficiency of 81.41%, an average particle diameter of 199.1nm, and a zeta potential of -31.2mV.
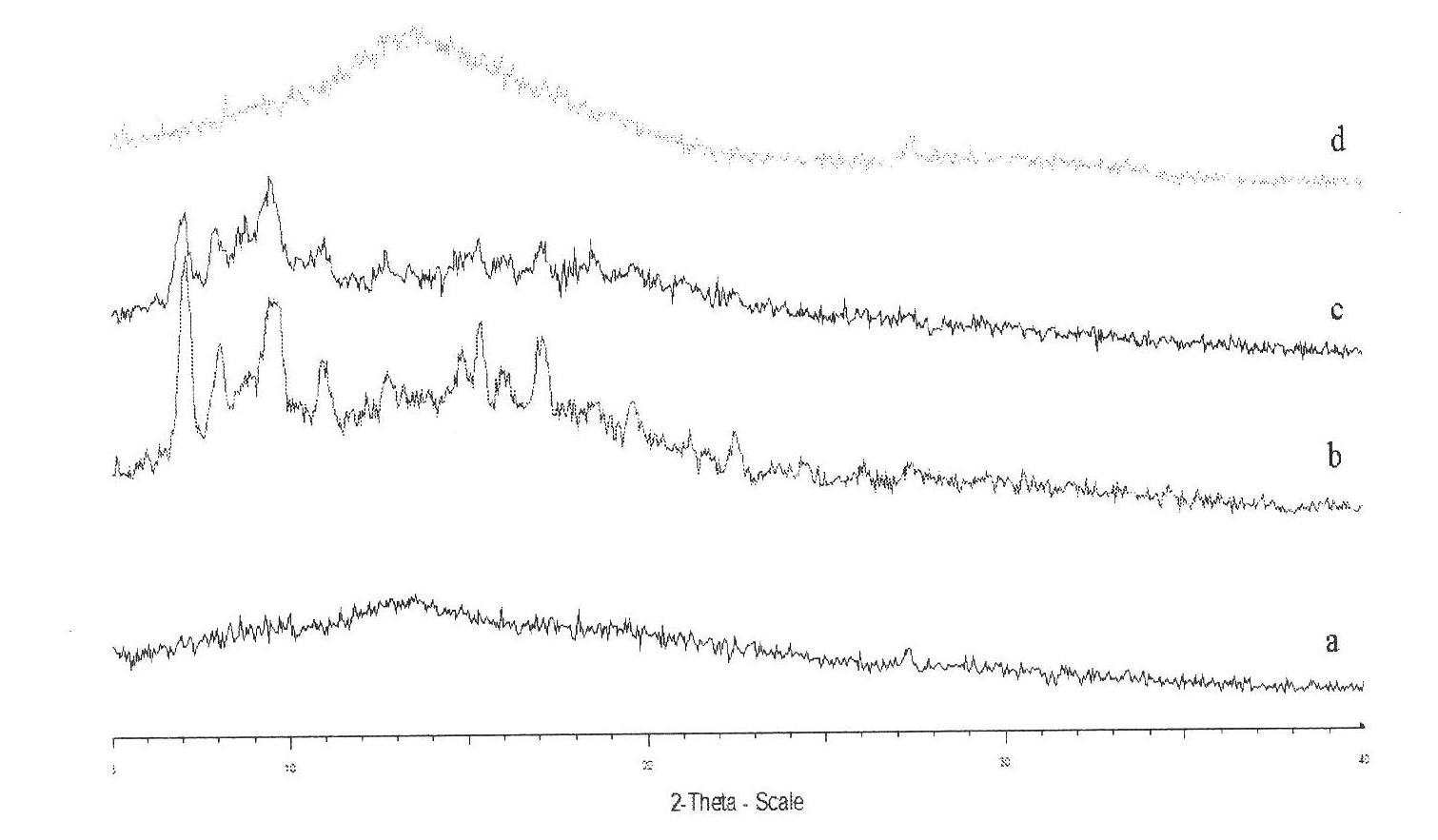
[0028] See attached figure 1 , figure 1It is the X-ray diffraction (XRD) pattern of various substances: wherein, curve a is an alkylated serum albumin derivative; curve b is cyclosporine A; curve c is cyclosporine A and alkylated serum albumin A physica...
Embodiment 2
[0031] Cyclosporine A Octyl Recombinant Human Serum Albumin Nanoparticles
[0032] Take 90mg of octyl-modified recombinant human serum albumin derivative, dissolve it in 15mL of double-distilled water, stir at room temperature for 0.5h to fully swell; dissolve 50mg of cyclosporine A in 1.25mL of absolute ethanol, and add it dropwise Medium, stirring; high-pressure homogenization (200bar, 2 times of circulation; 500bar, 2 times of circulation; 800bar, 3 times of circulation); dialysis bag (MWCO=12,000~14,000) single distilled water dialysis for 8h, the obtained nano solution passed through 0.45μm micropore filter membrane; add 1% (w / v) mannitol to the filtrate, mix evenly, subpackage, freeze-dry to obtain.
[0033] The test results show that the cyclosporin A octyl recombinant human serum albumin nanoparticles have a drug loading capacity of 30.48%, an encapsulation efficiency of 79.36%, an average particle diameter of 193.1nm, and a zeta potential of -33.0mV.
Embodiment 3
[0035] Cyclosporine A Octyl Porcine Serum Albumin Nanoparticles
[0036] Take 90mg of octyl-modified porcine serum albumin derivative, dissolve it in 15mL double-distilled water, stir at room temperature for 0.5h to fully swell; dissolve 40mg cyclosporin A in 1.0mL chloroform, add dropwise to the above carrier solution, stir Probe ultrasound (200w, 30min); Vacuum rotary evaporation for 2h to remove the organic solvent, the resulting nano-solution through a 0.45μm microporous membrane; add 1% (w / v) mannitol to the filtrate, mix well, sub-package, freeze-dry have to.
[0037] The test results show that the cyclosporin A octyl porcine serum albumin nanoparticles have a drug loading capacity of 24.61%, an encapsulation efficiency of 72.20%, an average particle diameter of 236.5nm, and a zeta potential of -33.6mV.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com