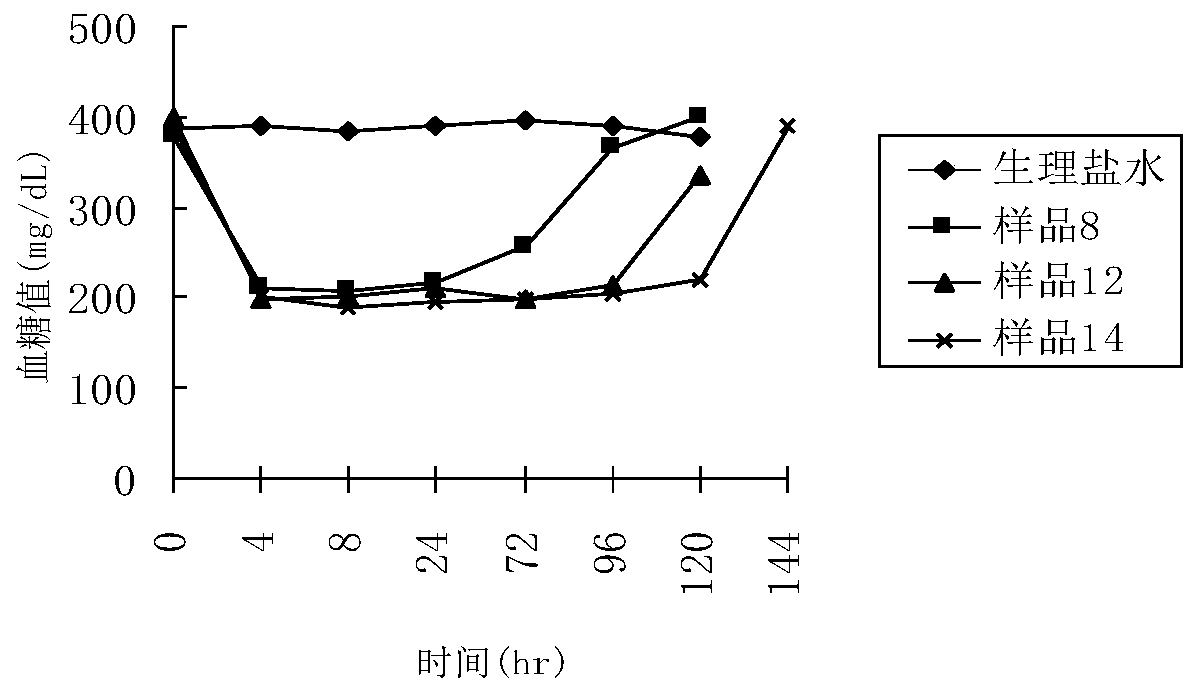
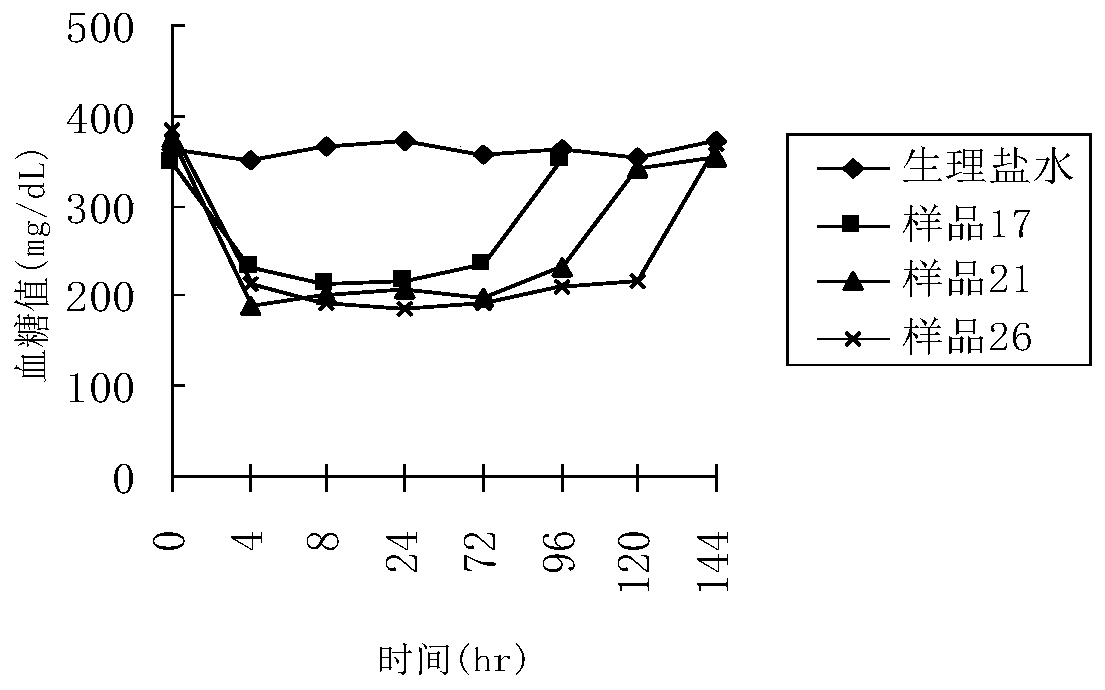
A polyethylene glycol-modified glucagon-like peptide-1 analogue
A technology of glucagon and polyethylene glycol, which is applied in the field of glucagon-like peptide-1 analogues, can solve problems such as drug resistance that cannot be solved, and achieve improved clinical application compliance, good metabolic stability, The effect of prolonging the half-life in vivo
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] The present invention will be further described below in conjunction with specific examples. This embodiment is only for explaining the present invention, and does not limit the content of the present invention in any way. For a better understanding of the present invention, see Table 1 for the abbreviations and Chinese names of the reagents used.
[0061] Table 1 Reagent abbreviation and Chinese name comparison
[0062]
[0063] Example 1
[0064] A. Preparation of glucagon-like peptide-1 analogs
[0065] 1) Synthesis: Using the Fmoc strategy, use the CS 336 peptide synthesizer (CS Bio) to synthesize step by step according to the following steps:
[0066] a) In the presence of an activator system, the Fmoc-amino acid-resin is obtained by coupling the resin solid phase carrier and the Fmoc-protected C-terminal amino acid; wherein, the synthesis of the C-terminal amidated polypeptide uses an amino resin, such as Rink Amide AM, Rink Amide , Rink MBHA et al.
[0...
Embodiment 2
[0072] Linear monomethyl PEG (21000 Daltons) modified SEQ ID NO:1
[0073] 1) Ligation: The polypeptide of SEQ ID NO:1 was dissolved in 50 mM sodium phosphate buffer solution at pH 6 containing 5 mM EDTA at a concentration of 2 mg / mL. Add 1.2-1.5 times molar amount of solid PEG-maleimide, stir to dissolve, and react at room temperature for 2 hours. The reaction was monitored by HPLC, terminated with 5 mM β-mercaptoethanol, and purified at room temperature for 30 min.
[0074] 2) Purification: Preparative ion-exchange column chromatography was used, filled with SP SepharoseHP, and eluted with a linear gradient of 0-500mM sodium chloride solution. The effluent was detected by HPLC and SDS-electrophoresis, and the PEG-polypeptide fraction was collected, concentrated by ultrafiltration or desalted with Sephadex G-25, etc., and freeze-dried.
[0075] The purity of the obtained PEG-modified polypeptide was tested by RP-HPLC, and the molecular weight was determined by MALDI-TOF.
Embodiment 3
[0077] Linear monomethyl PEG (30000 Daltons) modified SEQ ID NO:8
[0078] According to the method of Example 2, linear monomethyl PEG (30000 Daltons) modified SEQ ID NO: 8 and the following PEG conjugates of glucagon-like peptide-1 analogs were prepared.
[0079] Linear monomethoxy PEG (45000 Daltons) modified SEQ ID NO:7
[0080] Linear monomethoxy PEG (43000 Daltons) modified SEQ ID NO:8
[0081] Linear monomethoxy PEG (45000 Daltons) modified SEQ ID NO:9
[0082] Linear monomethoxy PEG (41000 Daltons) modified SEQ ID NO:10
[0083] Linear monomethoxy PEG (42000 Daltons) modified SEQ ID NO:11
[0084] Linear monomethoxy PEG (35000 Daltons) modified SEQ ID NO:13
[0085] Linear monomethoxy PEG (42000 Daltons) modified SEQ ID NO:14
[0086] Linear monomethoxy PEG (22000 Daltons) modified SEQ ID NO:16
[0087] Linear monomethoxy PEG (45000 Daltons) modified SEQ ID NO:18
[0088] Linear monomethoxy PEG (46000 Daltons) modified SEQ ID NO:19
[0089] Linear monomethoxy PEG ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com