Preparation and application of novel ghrelin-like peptide modification and dimerization
A technology for releasing hormones and growth hormones, which is applied in the field of medicine and biology to achieve the effect of long half-life and long-lasting activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Preparation of monomeric peptides and dimers
[0042] 1. Monomer peptide synthesis process: Manual solid-phase peptide synthesis operation steps.
[0043] 1. Resin swelling: put amino resin (amino resin for C-terminal amidation sequence) (purchased from Tianjin Nankai Synthetic Technology Co., Ltd.) into the reaction pot, add dichloromethane (DCM, Dikma Technologies Inc.) 15ml / g resin, shake for 30min. SYMPHONY 12-channel polypeptide synthesizer (SYMPHONY model, software Version.201, Protein Technologies Inc.).
[0044] 2. Connect the first amino acid: Remove the solvent through sand core suction filtration, add 3 times the mole of the first Fmoc-amino acid at the C-terminal (all Fmoc-amino acids are provided by Suzhou Tianma Pharmaceutical Group Fine Chemicals Co., Ltd.), and then add 10 Doubling molar amounts of 4-dimethylaminopyridine (DMAP) and N,N'-dicyclohexylcarbodiimide (DCC), and finally adding dimethylformamide (DMF) (purchased from Dikma Technol...
Embodiment 2
[0084] Example 2: Determination of Growth Hormone Release Activity
[0085] 1. Growth hormone (GH) release test in mice: Kunming mice (female, weighing 18-22 g, purchased from the Experimental Animal Center of Guangzhou University of Traditional Chinese Medicine, n=6). The regime for the care and use of all experimental animals was consistent with the Guidelines for Laboratory Animals. During feeding, the mice were reared alternately between light and dark for 12:12 hours, and the feeding temperature was 26±1°C. The peptide was subcutaneously injected into the buttocks of Kunming mice at a dose of 0.0133 μmol / kg, and blood was collected from the eyeballs 1 hour after administration, and serum was collected by centrifugation at 6000 rpm for 30 minutes for GH determination. In the experiment, all novel monomeric peptides or dimeric peptides were dissolved with T-NaCl-PB solution (detailed in the administration setting), and the negative control group was injected with T-NaCl-PB...
Embodiment 3
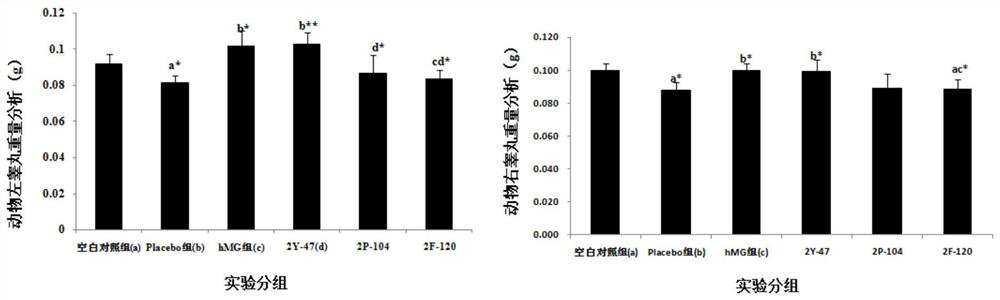
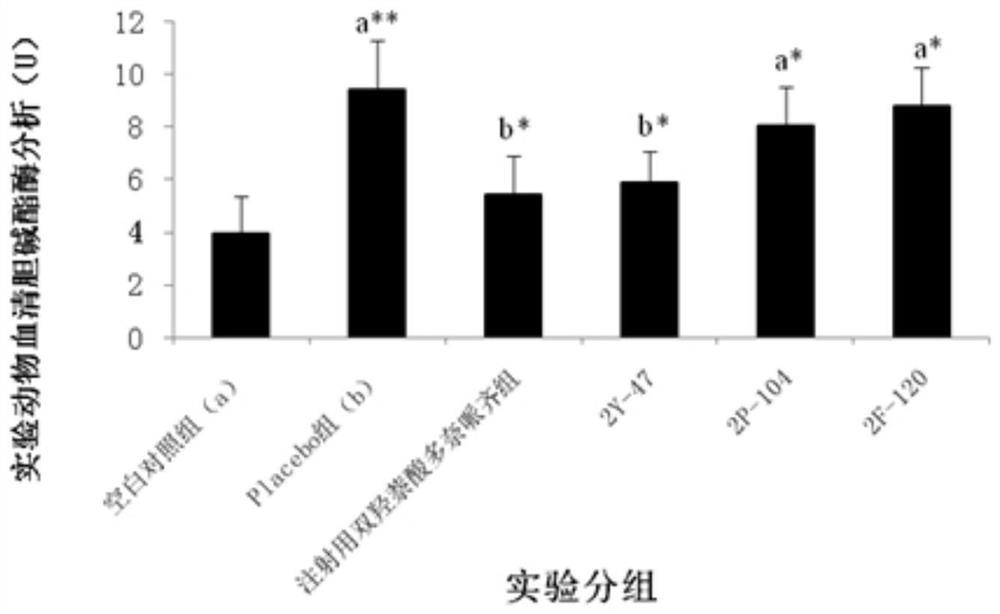
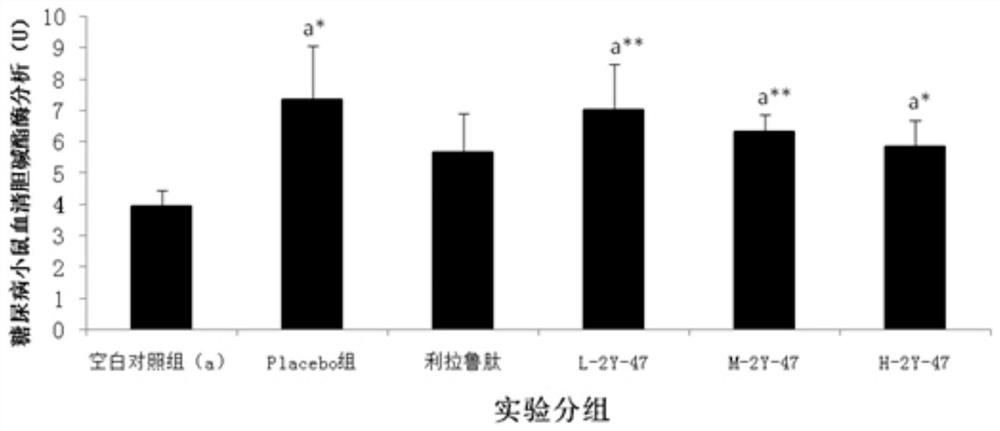
[0091] Example 3: Treatment of Dimeric Peptides to Infertility Models
[0092] 1. Infertility model test method: take 130 Chinese hamster males or 65 Chinese hamster females (five weeks old, 18-22g / only, provided by Sichuan Dashuo Experimental Animal Center. Because the preliminary experiment shows , the sensitivity of male mice to peptides is significantly weaker than that of female mice, so the infertility model of male mice is n=20, while the infertility model of female mice is n=10. Because the GHRH of Chinese hamsters has the highest 93% homology with humans), Divided into blank control group (20 male mice, 10 female mice) and model mice (110 male mice, 55 female mice). All model groups were intraperitoneally injected with cyclophosphamide (Jiangsu Hengrui Medicine Co., Ltd., National Drug Approval H32020857, batch number 12032925) at 20 mg / kg, once a week, five times in total. After injection of cyclophosphamide in the fourth week, the model mice were divided into model...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com