Toxoplasma gondii attenuated live vaccine with deficiency of OMPDC and LDH1 genes
A live attenuated vaccine, technology of Toxoplasma gondii, applied in the field of genetic engineering, can solve the problems of inability to replicate in vivo, loss of replication of Toxoplasma gondii, reduce pathogenicity, etc., to prevent acute, chronic and congenital infections, and improve immunity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
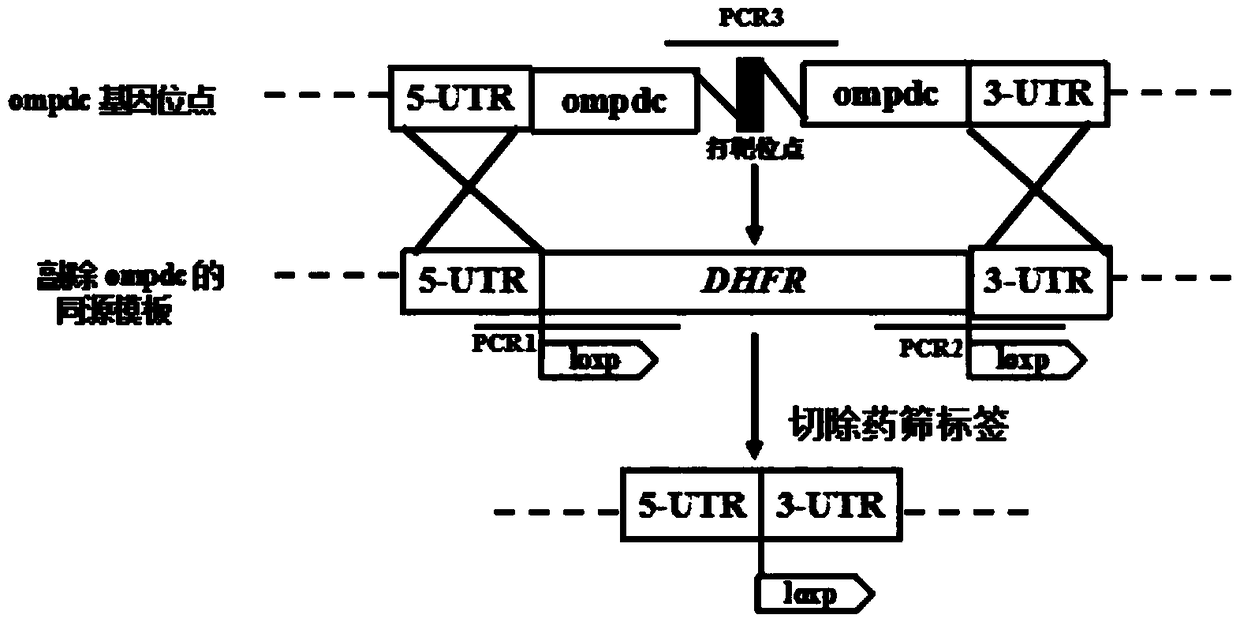
[0034] Example 1: Construction of Toxoplasma gondii auxotrophic strain △ompdc△ldh1
[0035] (1) Starting strain ME49
[0036] ME49 is a type II worm strain of the genus Toxoplasma of the family Toxoplasma in the order Coccidia, and has an orotidine-5-phosphate decarboxylase gene and a lactate dehydrogenase 1 gene, and the orotidine-5-phosphate decarboxylation The nucleotide sequences of the enzyme gene and the lactate dehydrogenase 1 gene are shown in SEQ ID NO: 1 and SEQ ID NO: 2, respectively.
[0037] (2) Construction of pSAG1-Cas9-TgU6-sgompdc plasmid
[0038] The pSAG1-Cas9-TgU6-sgompdc plasmid is the CRISPR plasmid of the CRISPR / Cas9 system. The plasmid was constructed using the pSAG1-Cas9-TgU6-ccdb-sgMIC3 plasmid as a template, using the NEB company Q5 point mutation kit ( Site-Directed Mutagenesis Kit) to replace the MIC3 target-specific gRNA with the gene ompdc target-specific gRNA, the specific operation steps are as follows:
[0039] ① Use gRNA to design the web...
Embodiment 2
[0131] Embodiment 2 Toxoplasma gondii △ ompdc △ ldh1 worm strain purposes
[0132] 2.1 Gene knockout strain △ompdc△ldh1 diluted injection
[0133] (1) Dilute injection formula
[0134]
[0135] (2) Preparation method of diluted injection
[0136] ① Mix the above-mentioned mixed solution with a fixed volume for 10 minutes with a magnetic stirrer;
[0137] ②Use a filter with a pore size of 0.22 μm to filter and sterilize in the ultra-clean bench.
[0138] 2.2 Mice toxicity test of △ompdc△ldh1 double knockout strain
[0139] 1) Use HFF cells to culture Toxoplasma gondii △ompdc△ldh1 double-knockout strain tachyzoites in vitro. After 20-30% of the parasites have escaped from the host cells, discard the medium in the original culture bottle and transfer it to the culture bottle. Add PBS to wash away residual escaping worms and medium, wash twice, and add the diluted injection prepared in step (1);
[0140] 2) Scrape off the cells with a disposable cell scraper, blow the susp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com