Indirect ELISA (enzyme-linked immunosorbent assay) detection kit based on toxoplasma gondii matrix protein 1
A detection kit and matrix protein technology, applied in the biological field, can solve problems such as difficult application in sick animals, and achieve the effects of good repeatability, good specificity, and easy source
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
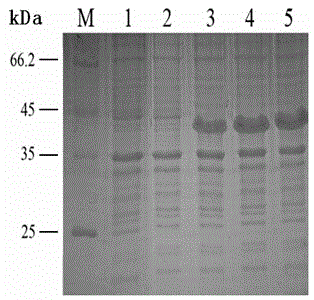
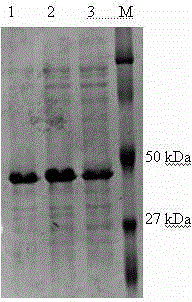
[0045] Example 1 Prokaryotic expression and purification of Toxoplasma gondii MAG1 protein
[0046] 1. Materials
[0047] 10× PCR Buffer, Taq Reagents required for PCR reactions such as DNA polymerase and dNTP, DNA Marker DL2000, and gel purification and recovery kit were purchased from Aisigen; Ni-FF affinity chromatography column was purchased from Beijing Weishi Bohui Chromatography Technology Co., Ltd.; restrictions endonuclease Kpn Ⅰ and EcoR Ⅰ, T4 DNA ligase, nucleic acid electrophoresis dye GoldView, purchased from Saibaisheng Biological Company; Biometra T-Gradient Thermoblock gradient PCR instrument, electrophoresis instrument, products of Beijing Liuyi Instrument Factory; gel imaging analysis system, Shanghai Peiqing Technology Co., Ltd.; constant temperature shaker, ultrasonic cracker, biochemical incubator, micro oscillator, pipette gun, pipette tip.
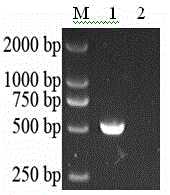
[0048] 2. Amplification and purification of MAG1 fragments
[0049]
[0050] Using the target gene ...
Embodiment 2
[0057] Example 2 Indirect ELISA detection technology specific detection results of Toxoplasma gondii MAG1 protein
[0058] 1. Materials
[0059] Biochemical incubator, micro shaker, wet box, pipette, NanoDrop ND-1000 UV spectrophotometer. Toxoplasma recombinant MAG1 protein, Toxoplasma canine positive serum, Toxoplasma canine negative serum and Escherichia coli, Salmonella, Listeria, canine parvovirus positive sera preserved in our laboratory. HRP enzyme-labeled SPA (dry powder purchased from Sangon Biotechnology Co., Ltd.), OPD, 30% H 2 o 2 solution, 0.2 M Na 2 HPO 4 solution, 0.1 M citric acid solution, skimmed milk (purchased from Guangming Dairy), Tween20, 2 M H 2 SO 4 solution, 0.01M PBS solution with pH 7.4.
[0060] 2. Dilution of recombinant protein
[0061] The MAG1 protein preserved in the laboratory was diluted to 15 μg / ml with 10 mM PBS at pH 9.6, and 100 μl per well was used for coating.
[0062] 3. Dilution of serum
[0063] Toxoplasma gondii canine p...
Embodiment 3
[0069] Example 3 Indirect ELISA Detection Technology Reproducibility Detection Results of Toxoplasma gondii MAG1 Protein
[0070] 1. Dilution of Positive Sera
[0071] Take 14 canine serum, dilute 1:50 with 10 mM PBS, pH 7.4 containing 5% skimmed milk powder, and use it as the primary antibody.
[0072] 2. Repeatability detection
[0073] Method is the same as embodiment one. Take 7 diluted sera, do 4 tests for 4 consecutive days, compare the differences in the test results, and calculate the coefficient of difference between the plates; take another 7 diluted sera, and do 3 replicates of each serum on the same plate, compare The difference between the results was calculated by calculating the coefficient of variation within the plate.
[0074] 3. Test results
[0075] The test results are shown in Table 3 and Table 4. The coefficient of variation between plates is less than 3%, and the coefficient of difference within the plate is less than 10%. This method has good rep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com