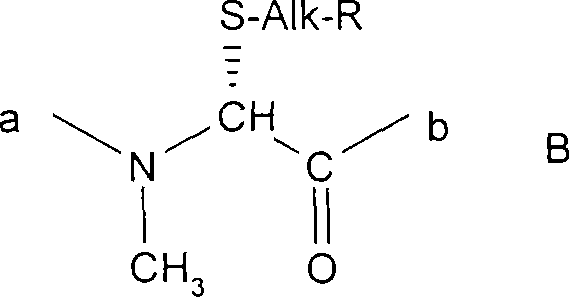
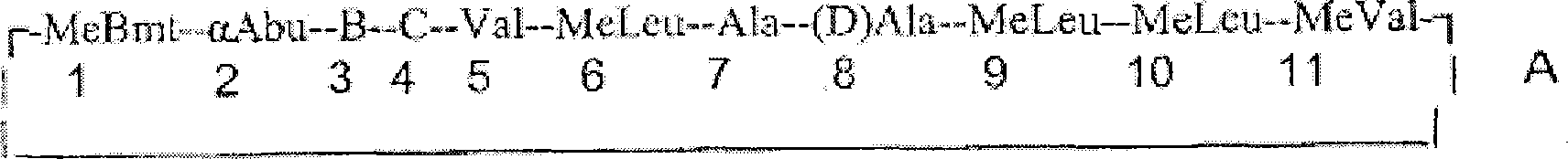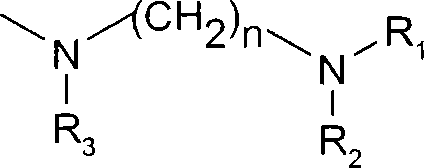Cyclosporins to treat alzheimer's disease
A technology for Alzheimer's disease and cyclosporine, applied in the direction of cyclosporine, cyclic peptide components, nervous system diseases, etc., can solve the problem of insufficient immunosuppressive activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0124] Presenilin Processing: Overexpression of cDNA was observed to increase Aβ levels of C99 substrates, suggesting increased GACE-mediated APP cleavage. Since CPZ and CyD significantly enhanced the processing of C99 and increased Aβ42 secretion, they were selected for further study. PS1 N-terminal fragment (NTF) levels in HEK293 cells transfected with CPZ or CyD were detected. In cells stably transfected with PS1, full-length PS1 and NTF can be readily detected. In contrast, endogenous full-length PS1 and NTF levels were lower in untransfected HEK cells. Significant increases in PS1 NTF levels in cells overexpressing CPZ or CyD indicated that these fragments were produced at a higher rate or that endogenous NTFs were stabilized during the 48 hours of transfection.
[0125] CPZ Analysis: CPZ is a member of the carboxypeptidase (CP) gene family, belonging to the CPE subfamily, known to process bioactive neuropeptides (Song and Fricker 1997). CPE-associated enzymes are ofte...
Embodiment 2
[0136] C99 and Notch cleavage
[0137] HEK 293 stable cell lines were generated using modified C99 or Notch transmembrane sequences with signal peptide, TGN retention sequence, and insertion of the GAL4-NLS-VP16 sequence at Q56 / Y57 as described by Maltrese, Wilson et al. 2001 . The cells also stably expressed the 5xGAL4RE-luciferase reporter gene constructs (RD-2002-01437 and RD-2001-02419). GACE activity in these cells was measured when the C99 transgene was cleaved, thereby activating the Gal4 luciferase reporter gene. These HEK stable cells were treated with cyclosporin A, Sangliferhin A, FK506, or N-methyl-4-valine-cyclosporine and the IC for C99 cleavage was determined 50 . GALVP alone can be used as a negative control. GALVP IC for cyclosporin A, N-methyl-4-valine-cyclosporine, Sangliferhin A and FK506 50 6.9, 9.9, >20, and >20 μM, respectively, suggesting a clear link between inhibition of C99 cleavage and cell viability as these compounds were not toxic until >40 ...
Embodiment 3
[0139] Caspase-3 activation
[0140] Recent studies suggest that activation of caspase-3 can lead to increased Aβ secretion and stabilization of proteins in the GACE complex (Tesco, Koh et al. 2003). Since both CyD and CPZ enhance Aβ secretion and stabilize PS1 NTF, the level of caspase-3 activation was analyzed in CyD and CPZ overexpressing cells. HEK 293 cells were transiently transfected with CyD and CPZ together with APPwt for 24 hours, saponin permeabilized, fixed and probed with FITC-conjugated monoclonal antibody against active caspase-3. Negative control cells were transfected with empty vector and APPwt, while positive control cells were treated with 1 μM staurosporine for 6 hr before analysis. In cells expressing CyD, 52% of the cells tested were positive for active caspase-3, whereas in cells expressing CPZ, 48% of cells were positive for caspase-3. These results indicate that overexpression of these proteins induces the activation of caspase-3, suggesting this as...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com