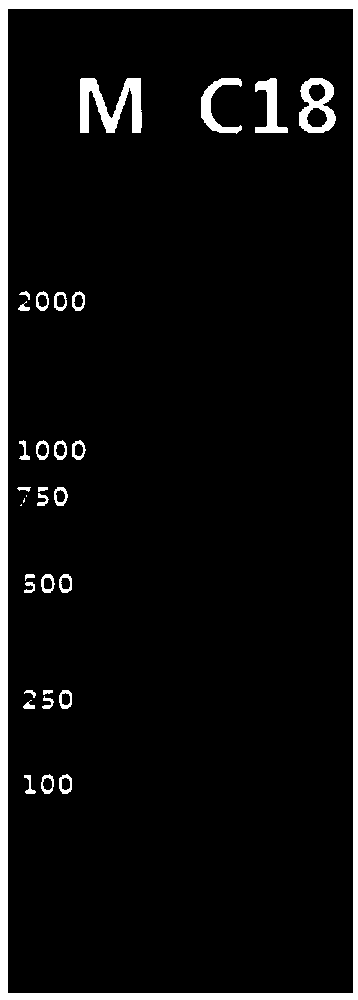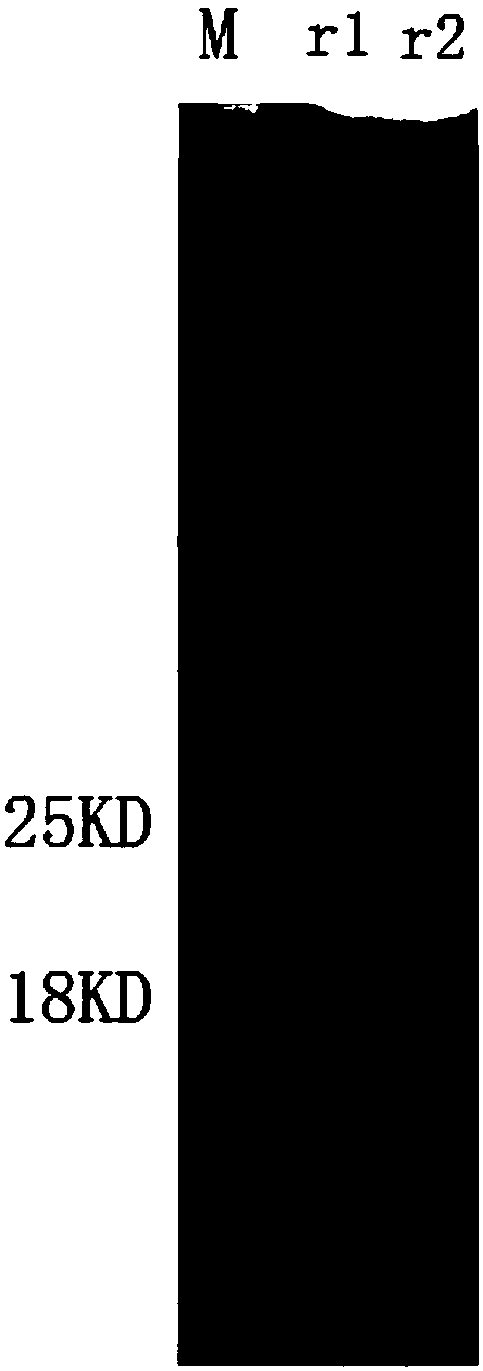Vaccine for preventing dog Toxoplasma gondii infection and preparation method of vaccine
A technology for toxoplasmosis and prevention of dogs, applied in the fields of immunology and biology, can solve the problems of vaccines and drugs for canine toxoplasmosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1: Construction of the prokaryotic expression vector of Toxoplasma gondii recombinant cyclophilin mutant protein
[0061] The gene is artificially synthesized according to the nucleic acid sequence SEQ ID NO.1, and primers are designed according to the physical map of the prokaryotic expression vector pET28a and restriction sites are introduced.
[0062] Upstream primer: ATGGAATTC ATGGAAAACGCGGGCGTGCGCAAA
[0063] Downstream primer: AAGCTT TTCTTTTTTGCCAATATCGGTAA
[0064] Synthesize the gene sequence of SEQ ID NO.1 by artificial synthesis method, use the above primers to amplify in large quantities, and clone the PCR purified product into pMD-18-T. The positive clones are identified by enzyme digestion and sequencing, and the positive plasmids are detected by EcoRI and Hind III The purified target fragment after double enzyme digestion was connected to the prokaryotic expression vector pET28a, the ligation product was transformed into E.coli DH5α competent c...
Embodiment 2
[0065] Embodiment 2: Purification of expressed protein
[0066] (1) Extraction and solubility verification of expressed protein
[0067] A single colony of E.coli BL21(DE3) transformed with the recombinant plasmid pET-28a(+)-C18 was inoculated in 5mL LB liquid medium and cultured overnight at 37°C with shaking. The next day, the seed liquid was transferred to 800mL LB liquid medium for propagation at 37°C, and the expression was induced under the optimal conditions when the OD600 of the bacterial liquid was monitored to 0.6-0.7. The bacteria were collected by centrifugation, suspended in 20mL PBS, frozen and thawed 5 times (-80°C, 1h / 37°C, 10min), after sonication, the supernatant was centrifuged to obtain the soluble protein (active protein). Suspend the precipitate with 10 mL of denaturing buffer, shake at room temperature for 1 h, and centrifuge to obtain the supernatant, which is the insoluble protein (denatured protein). The above two proteins were tested by SDS-PAGE to...
Embodiment 3
[0070] Example 3: Detection of the immunogenicity of expression products to mouse macrophages
[0071] RAW264.7 cells were seeded on 24-well culture plates at 0.5×106 / mL, 0.5 mL per well, 5% CO2, and cultured at 37°C for 24 hours. After aspirating the supernatant, dissolve the prepared Toxoplasma gondii recombinant cyclophilin mutant protein in RAW264.7 cell culture medium, and add 0.5 mL / well to 24 In the well plate, an equal volume of PBS was used as a negative control. The cell culture plate was placed in 5% CO2, cultured in a 37° C. incubator for 48 hours, the supernatant was collected, and the level of TNF-a was detected by ELISA experiment. The results show that the production of TNF-a increases with the increase of protein concentration, ranging from 40pg-310pg, the results are shown in the appendix Figure 5 . It shows that the recombinant cyclophilin mutant protein of Toxoplasma gondii can stimulate RAW264.7 cells to produce TNF-a.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com