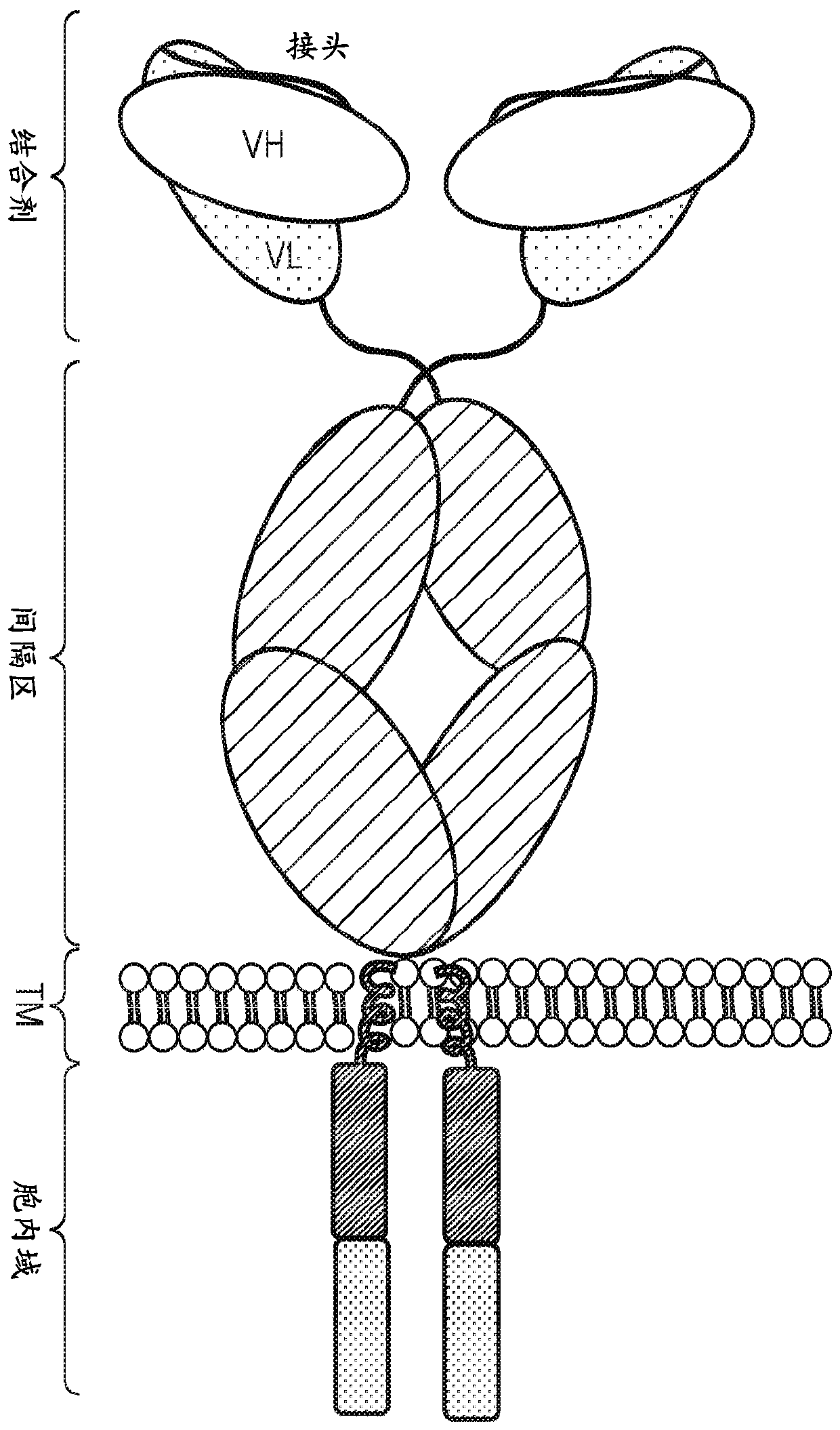
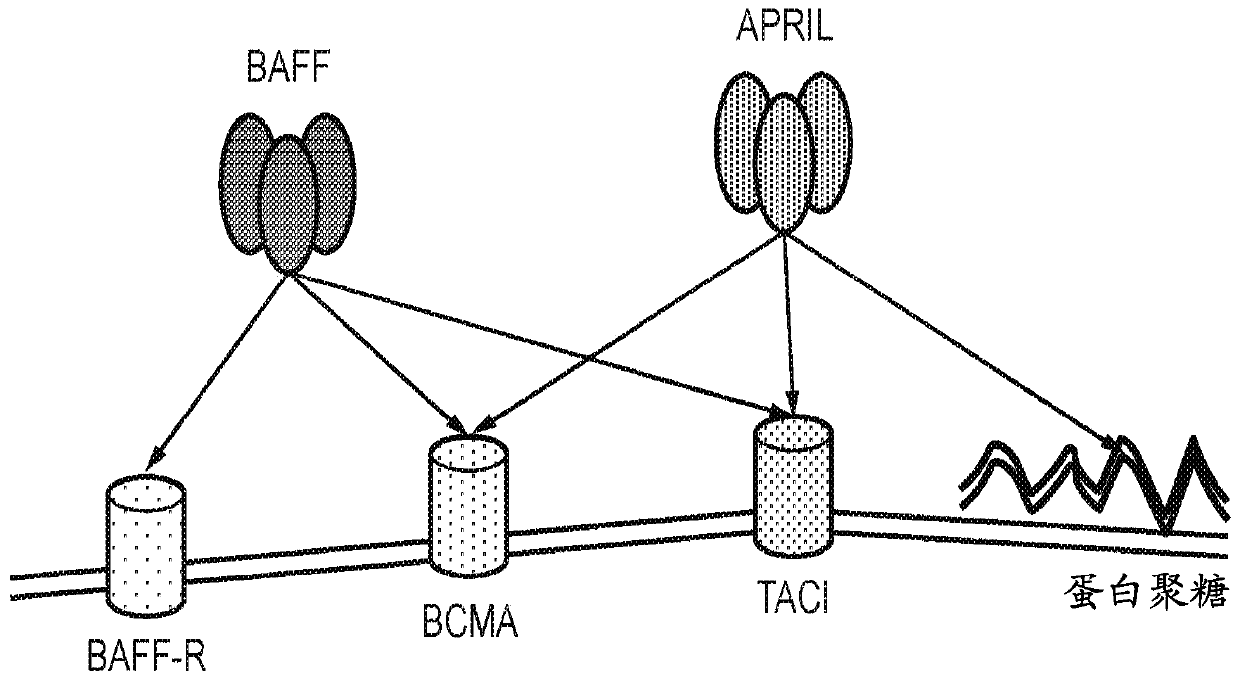
Chimeric antigen receptor
An antigen and ligand technology, applied in antibody medical components, fusion with soluble cell surface receptors, drug combinations, etc., can solve problems such as failure to cause long-term remission and loss of CD20 expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0414] Example 1 - Expression of BCMA on the surface of myeloma cells
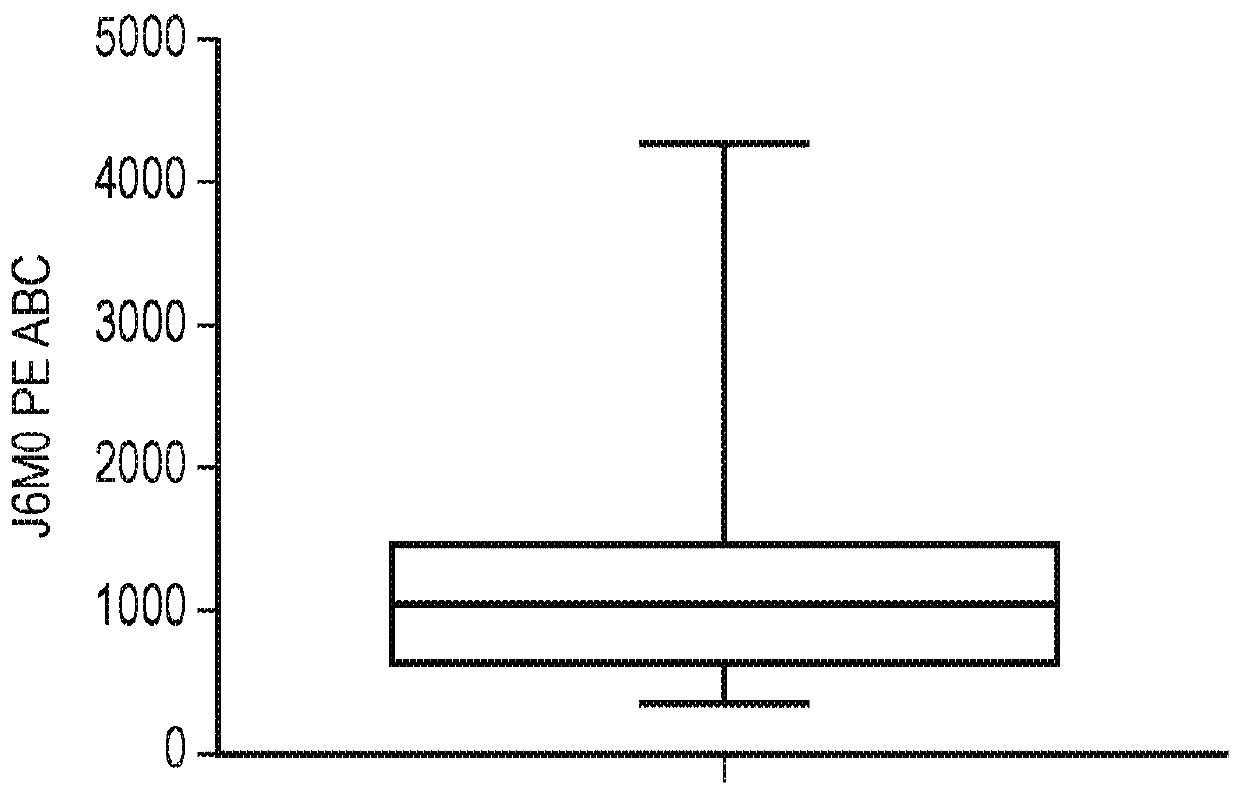
[0415] Primary myeloma cells were isolated by CD138 immunomagnetic selection on fresh bone marrow samples from multiple myeloma patients known to have overt disease. These cells were stained with BCMA-specific J6MO mAb (GSK) conjugated to PE. In parallel, a standard of beads with a known number of binding sites was generated using the PE Quantibrite Bead Kit (Becton Dickenson) following the manufacturer's instructions. BCMA copy numbers on myeloma cells were obtained by correlating the mean fluorescence intensity from myeloma cells with a standard curve derived from the beads. The range of BCMA copy numbers on the surface of myeloma cells was found to be low: 348.7-4268.4 BCMA copies per cell, with a mean of 1181 and a median of 1084.9 ( figure 2 ). This is much lower than eg CD19 and GD2 (classic targets of CARs).
Embodiment 2
[0416] Example 2 - Design and Generation of TACI CAR
[0417] Immunization and hybridoma generation
[0418] The gene encoding human TACI was cloned in the vector pVAC2. Five BalBC mice were immunized with plasmid DNA encoding TACI adsorbed to gold nanoparticles. Using Gene-Gun TM (Biorad) system for intramuscular delivery of coated gold nanoparticles. Mice were boosted 3 times over the course of 21 days. Test blood from mice was screened for anti-TACI antibody titers by ELISA and flow cytometry.
[0419] Five mice with TACI-positive sera were selected for a final immune boost, and then spleens were harvested for B-cell isolation and hybridoma production. Hybridoma fusions using 10 x 96-well plates of lymphocytes from selected mice were performed. Hybridoma supernatants were screened for reactive anti-TACI antibodies by ELISA screening against recombinant human TACI purified protein (Peprotech, 310-17), representative data are shown in Figure 6a middle. ELISA positi...
Embodiment 3
[0427] Example 3 - Killing of target cells by TACI-CAR T cells
[0428] Retroviruses were generated by transiently transfecting 293T cells with plasmids encoding CAR, gag / pol, and the envelope protein RD114. After 3 days, the supernatant was harvested and used to transduce PHA / IL2-activated PBMCs at the same titer as the retrovirus on retronectin-coated plates. CAR expression was confirmed by flow cytometry 6 days after transduction, and PBMCs were co-cultured with TACI+BFP SupT1 cells or BCMA+BFP SupT1 cells at a ratio of 1:1. Target cell killing was determined after one and three days. Also after one and three days, supernatants were removed and interferon-γ levels were determined by ELISA. The result is as Figure 7 Shown in b and c.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com