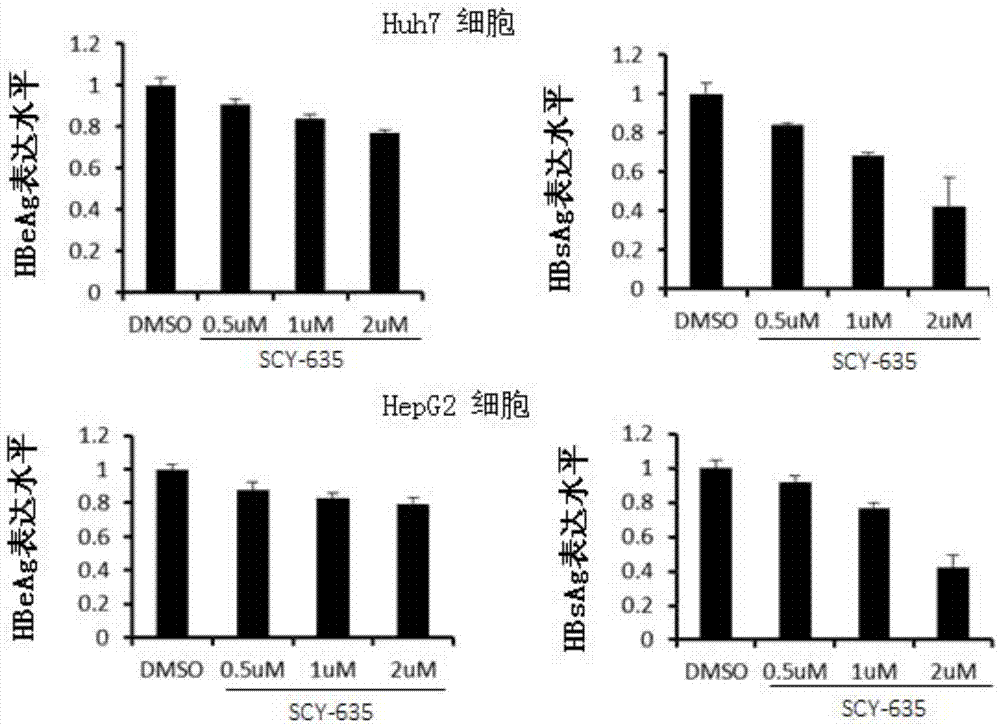
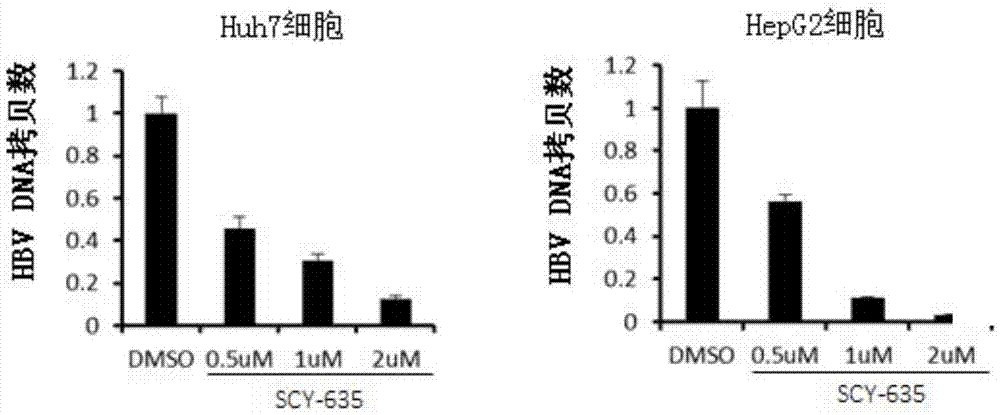
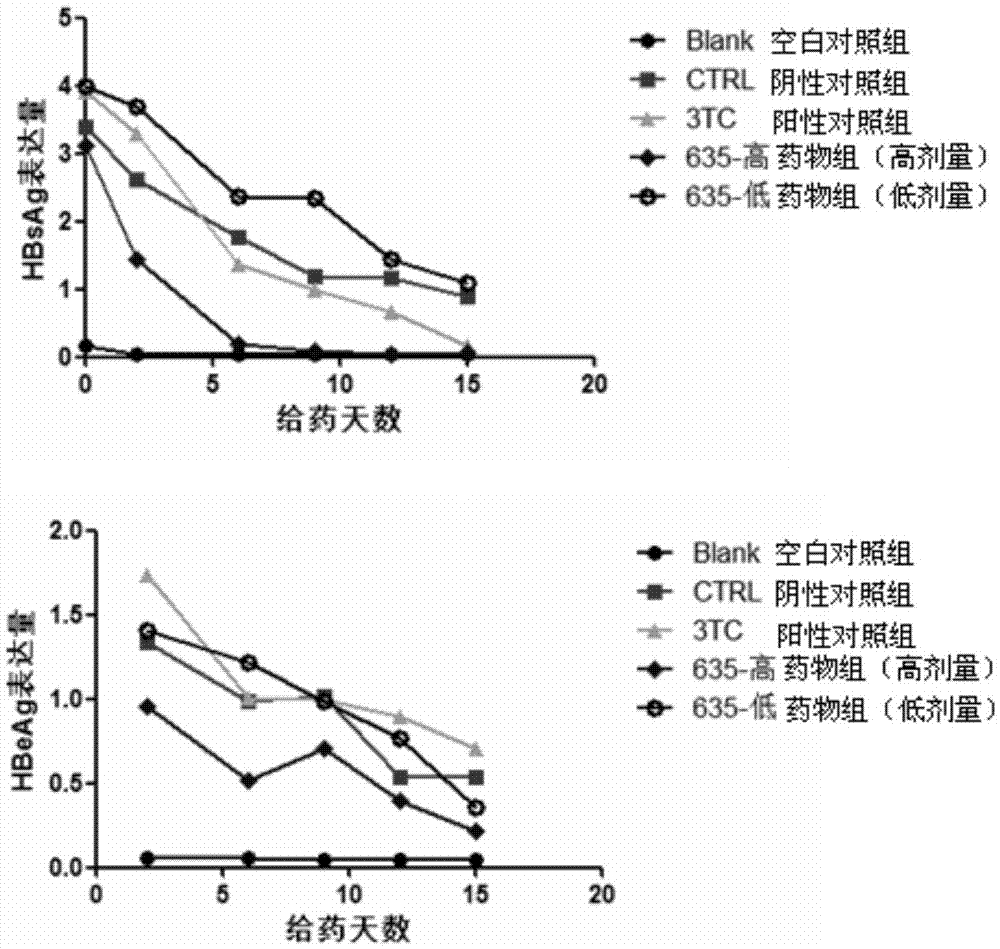
Application of cyclophilin inhibitor
A cyclosporine and inhibitor technology, applied in the field of cyclophilin inhibitors, can solve the problems of insufficient inhibitory effect and weak anti-hepatitis B virus efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Example 1 Preparation of 3-methoxycyclosporine
[0087] A solution of 3-(mercaptobenzothiazol-2-ylthio)cyclosporin (0.4 g, 0.28 mmol) and camphorsulfonic acid (0.7 g, 3 mmol) in dry tetrahydrofuran and dry methanol was heated at 50 °C for 2 h. The mixture was cooled to room temperature and saturated sodium bicarbonate, ether and water were added. The layers were separated and the aqueous phase was extracted with diethyl ether. The combined organic extracts were dried over anhydrous magnesium sulfate and filtered. Chromatography was repeated on silica gel, eluting with a mixture of dichloromethane and ethyl acetate, to obtain 120 mg of 3-methoxycyclosporin.
Embodiment 2
[0088] Example 2 Preparation of 3-(2-methoxyethylthio)-4-(γ-hydroxymethylleucine) cyclosporine
[0089] The liquid ammonia (30 mL) was concentrated under nitrogen. Sodium amide (1.0 g) was added followed by a solution of 4-(γ-hydroxymethylleucine)-cyclosporin (1.22 g, 1.0 mmol) in tert-butyl methyl ether (20 mL). The mixture was stirred at -35°C for 90 minutes. 2-Methoxyethyl disulfide (5.9 g) was added and stirring was continued for a further 2 hours at -35°C. Solid ammonium chloride (1.5 g) was added and the mixture was stirred at -33°C for 10 minutes. After returning to room temperature, the mixture was diluted with tert-butyl methyl ether, washed with water, brine, and dried over anhydrous sodium sulfate. After removal of the solvent, the residue was purified using silica gel column chromatography, eluting first with ethyl acetate / heptane and then methanol / ethyl acetate to obtain 500 mg of 3-(2-methoxyethylthio)-4 -(γ-hydroxymethylleucine)cyclosporine.
Embodiment 3
[0090]Example 3 Preparation of 3-[(R)-2-(N,N-dimethylamino)ethylthio-Sar]-4-(γ-hydroxymethylleucine)cyclosporine
[0091] In an inert atmosphere, 4-(γ-hydroxymethylleucine) cyclosporine (1.22 g, 1.0 mmol) was added dropwise to a solution of lithium diisopropylamide (LDA) in dry tetrahydrofuran at low temperature. The tetrahydrofuran solution was dried. Stirring of the mixture was continued at -35°C for 90 minutes. 2-(N,N-Dimethylamino)ethyl disulfide (4.6 g) was added and stirring was continued for a further 2 hours at -35°C. Solid ammonium chloride (1.5 g) was added and the mixture was stirred at -33°C for 10 minutes. After returning to room temperature, the mixture was diluted with tert-butyl methyl ether, washed with water, brine, and dried over anhydrous sodium sulfate. After removal of the solvent, the residue was purified using silica gel column chromatography, eluting first with ethyl acetate / heptane and then with methanol / ethyl acetate to obtain 260 mg of 3-[(R)-2-(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com