Fluorescence quantitative PCR detection kit of hepatitis B virus and application thereof
A hepatitis B virus and detection kit technology, applied in the direction of fluorescence/phosphorescence, microbial measurement/inspection, biochemical equipment and methods, etc., can solve the problems of large sample demand, narrow quantitative range, and difficulty, and achieve The effect of reducing personnel operation errors, improving detection sensitivity, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
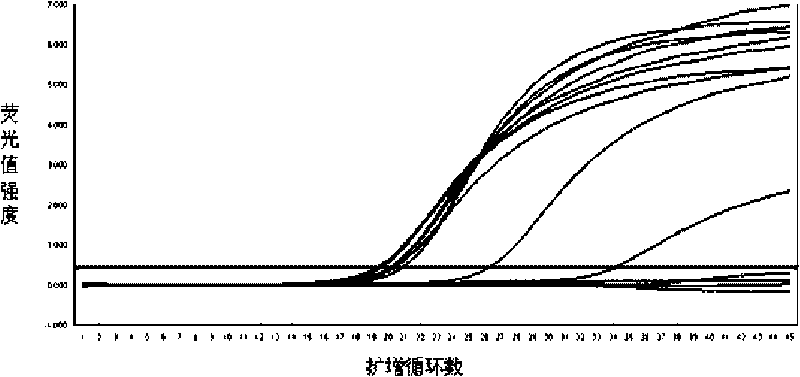
[0050] Embodiment 1: specificity test
[0051] The known HBV-positive samples and HBV-negative samples were used for detection to examine the specificity of the kit. The formulations and operating procedures of each reagent are as follows:
[0052] Reagent preparation:
[0053] DNA extraction solution I: containing sodium lauryl sulfate 0.2% (mass / volume), triton 1.0% (volume / volume), guanidine isothiocyanate 0.2mol / L;
[0054] DNA extraction solution II: containing 4-hydroxyethylpiperazineethanesulfonic acid 100mmol / L, pH6.3, sodium chloride 100mmol / L, magnetic beads 100μg / ml;
[0055] DNA extraction solution III: containing Triton 0.1% (volume / volume), sodium chloride 100mmol / L;
[0056] DNA extraction solution IV: mineral oil;
[0057] The internal standard (positive internal control) is: a recombinant of a 97-base-pair artificially synthesized DNA sequence inserted into the pUC18T vector, that is, a plasmid (hereinafter referred to as a recombinant plasmid), with a con...
Embodiment 2
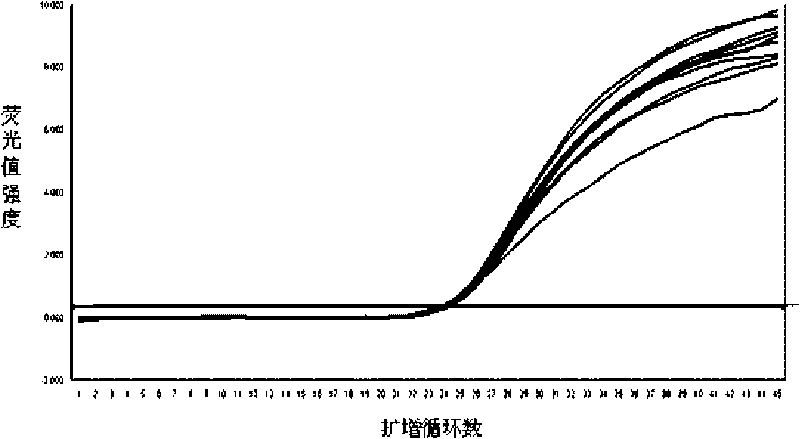
[0085] Embodiment 2: The impact of different extraction methods of DNA on the detection of HBV
[0086] Existing a lot of mature DNA extraction kits on the market can be used for the extraction of HBV-DNA in the sample at present, select common boiling cracking method and column passing method to compare with the magnetic bead method extraction nucleic acid in the kit of the present invention, select high, Eight cases of HBV-positive samples (including serum, plasma and breast milk) with medium and low concentrations were subjected to fluorescent PCR reaction after extracting DNA respectively. The reagent formula and detection steps are as follows:
[0087] Reagent preparation:
[0088] The formula of DNA extraction solution I: sodium lauryl sulfate 0.5%, triton 2.5%, guanidine isothiocyanate 0.6mol / L;
[0089] The formula of DNA extraction solution II: 4-hydroxyethylpiperazineethanesulfonic acid 200mmol / L, pH6.5, sodium chloride 200mmol / L, magnetic beads 250μg / ml;
[0090]...
Embodiment 3
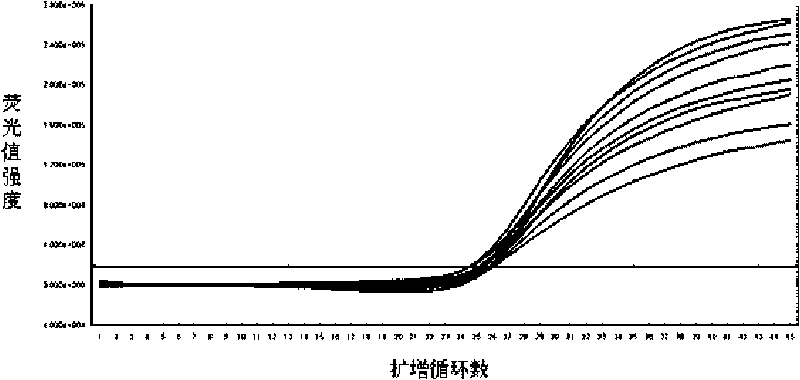
[0135] Example 3 Fluorescence normalization correction
[0136] Due to the unavoidable accidental factors such as the error of sample loading operation, the difference in the light transmission performance of the centrifuge tube, and the difference in the fluorescence excitation efficiency, the original signal collected by the instrument must be normalized to eliminate the influence of these factors on the test results. This correction can be achieved by adding additional fluorescent dyes (called reference fluorescence) in the reaction system, such as adding ROX reference fluorescence, the concentration of ROX in the PCR reaction solution is constant, so the intensity of its signal is related to the reaction The volume of the system is positively correlated with the fluorescence excitation efficiency. When the reaction volume changes or the excitation light efficiency changes, the ROX signal and the signal of the target gene (such as FAM) are affected to the same degree (assumi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com