TACI-immunoglobulin fusion proteins
An immunoglobulin and fusion protein technology, applied in the field of improved TACI-immunoglobulin fusion protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
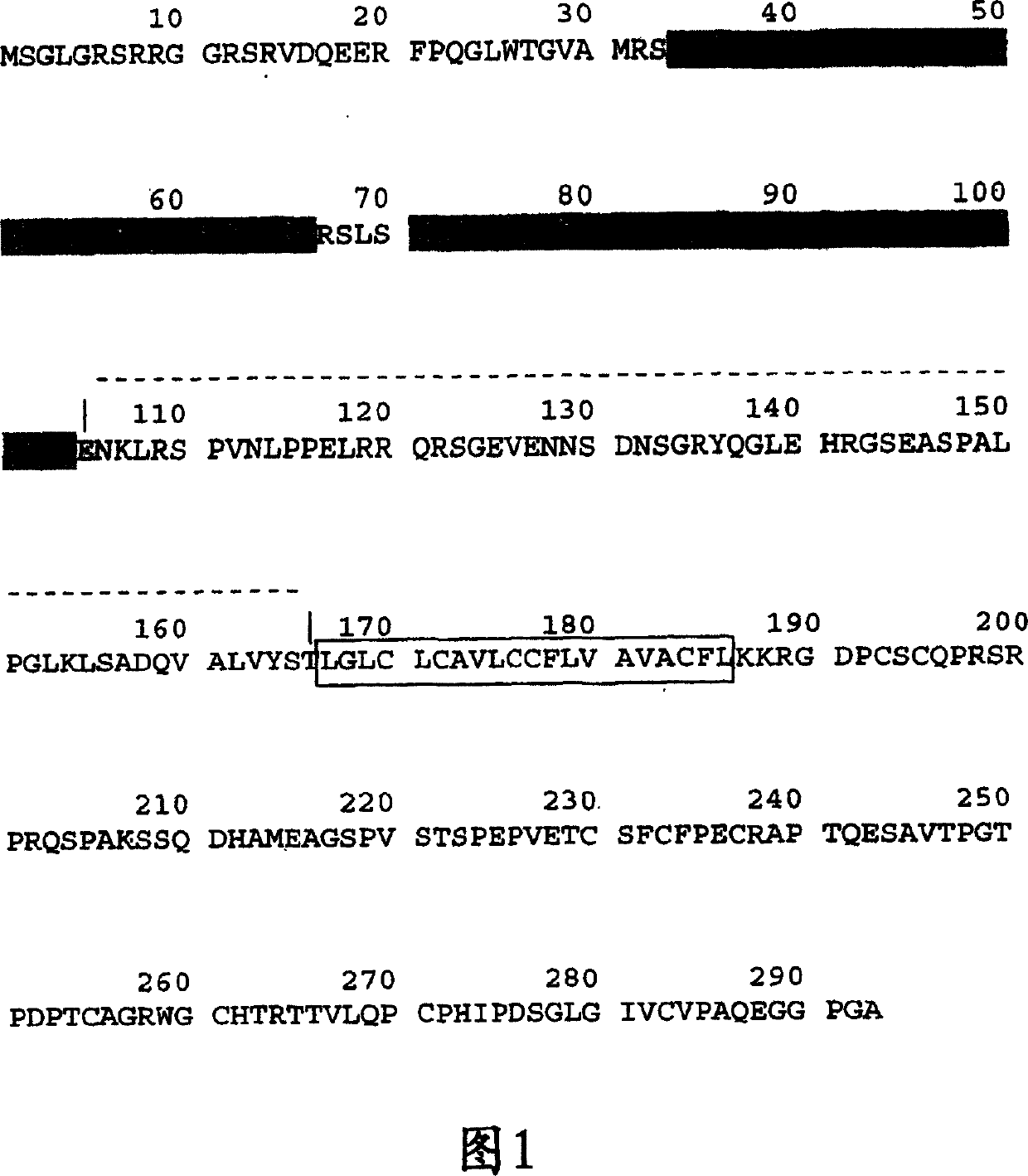
[0137] Example 1 describes an expression vector comprising a cytomegalovirus promoter directing transgenic expression of a recombinant protein, an immunoglobulin intron, and a tissue plasminogen activator signal sequence. A suitable immunoglobulin intron is the murine 26-10V H Intron. SEQ ID NO: 66 provides mouse 26-10V H Exemplary nucleotide sequences of introns. The expression vector may also include a 5' untranslated region (UTR) upstream of the nucleotide sequence encoding the TACI-immunoglobulin protein. Suitable 5'-UTR can be from mouse 26-10V H obtained in the gene. SEQ ID NO: 63 discloses useful natural mouse 26-10V H The 5'-UTR nucleotide sequence, while SEQ ID NO: 64 shows the mouse 26-10V H When using the 5'-UTR nucleotide sequence, it has been optimized at the 3' end.
[0138] As an example, SEQ ID NO: 67 provides the nucleotide sequence comprising the following elements: native murine 26-10V H 5'-UTR (nucleotides 1 to 51), mouse 26-10V H Signal sequence...
Embodiment 2
[0295] Production of TACI-Fc Protein by Chinese Hamster Ovary Cells
[0296] By means of electroporation, the TACI-Fc expression construct was used to transfect suspension-adapted Chinese hamster ovary (CHO) DG44 cells grown in animal protein-free medium (Urlaub et al., Som. Cell. Molec. Genet. 12:555 (1986)). CHO DG44 cells lack a functional dihydrofolate reductase gene due to deletions at two dihydrofolate reductase chromosomal locations. Growth of the cells in the presence of increasing concentrations of methotrexate results in amplification of the dihydrofolate reductase gene and the linked gene encoding the recombinant protein on the expression construct.
[0297] CHO DG44 cells were passaged in PFCHO medium (JRH Biosciences, Lenexa, KS), 4mM L-glutamine (JRH Biosciences), and 1x hypothanxine-thymidine rehydration solution (Life Technologies), and then incubated at 37°C and 5% CO 2 The cells were incubated in Corning shaker flasks on a rotary shaker platform r...
Embodiment 3
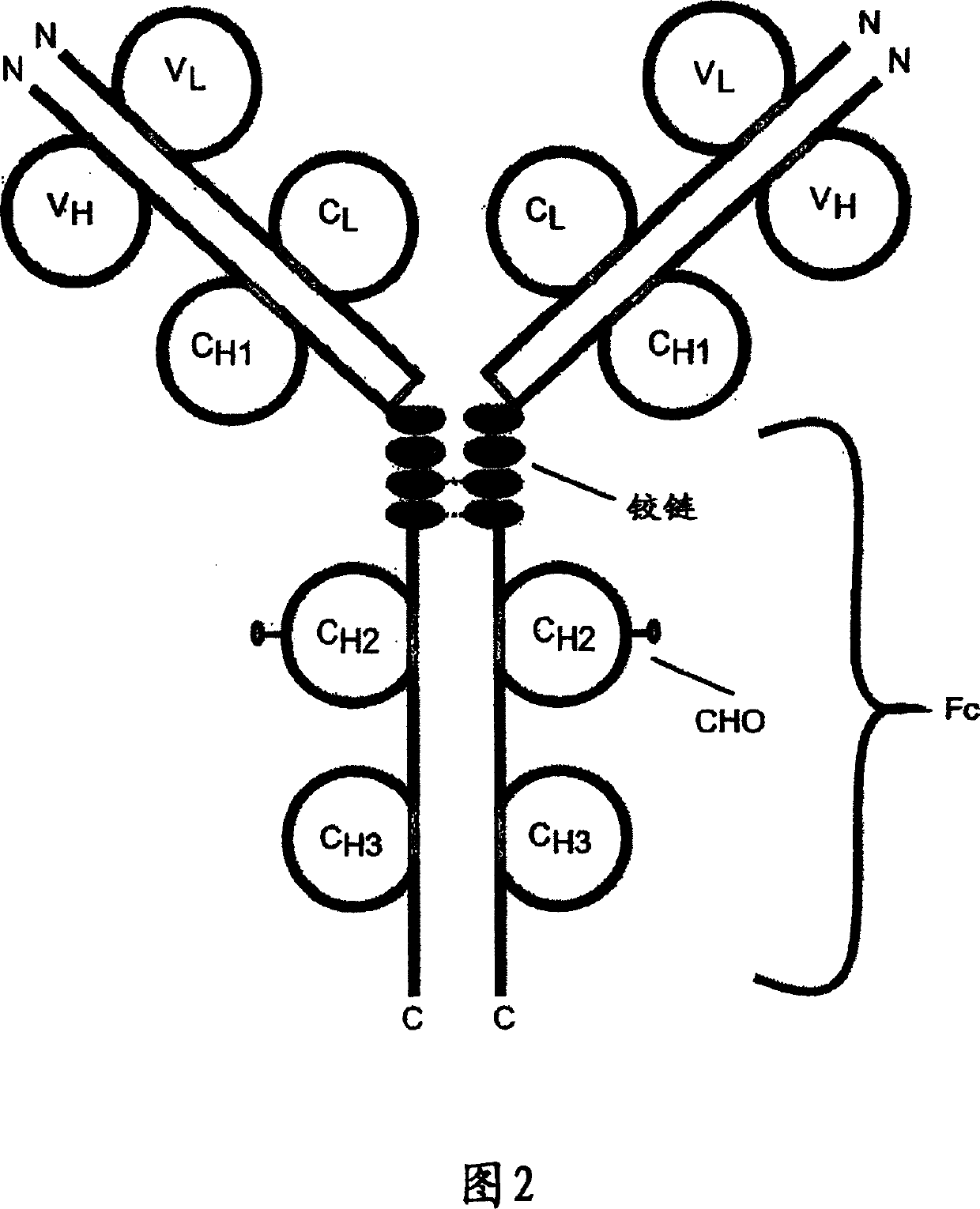
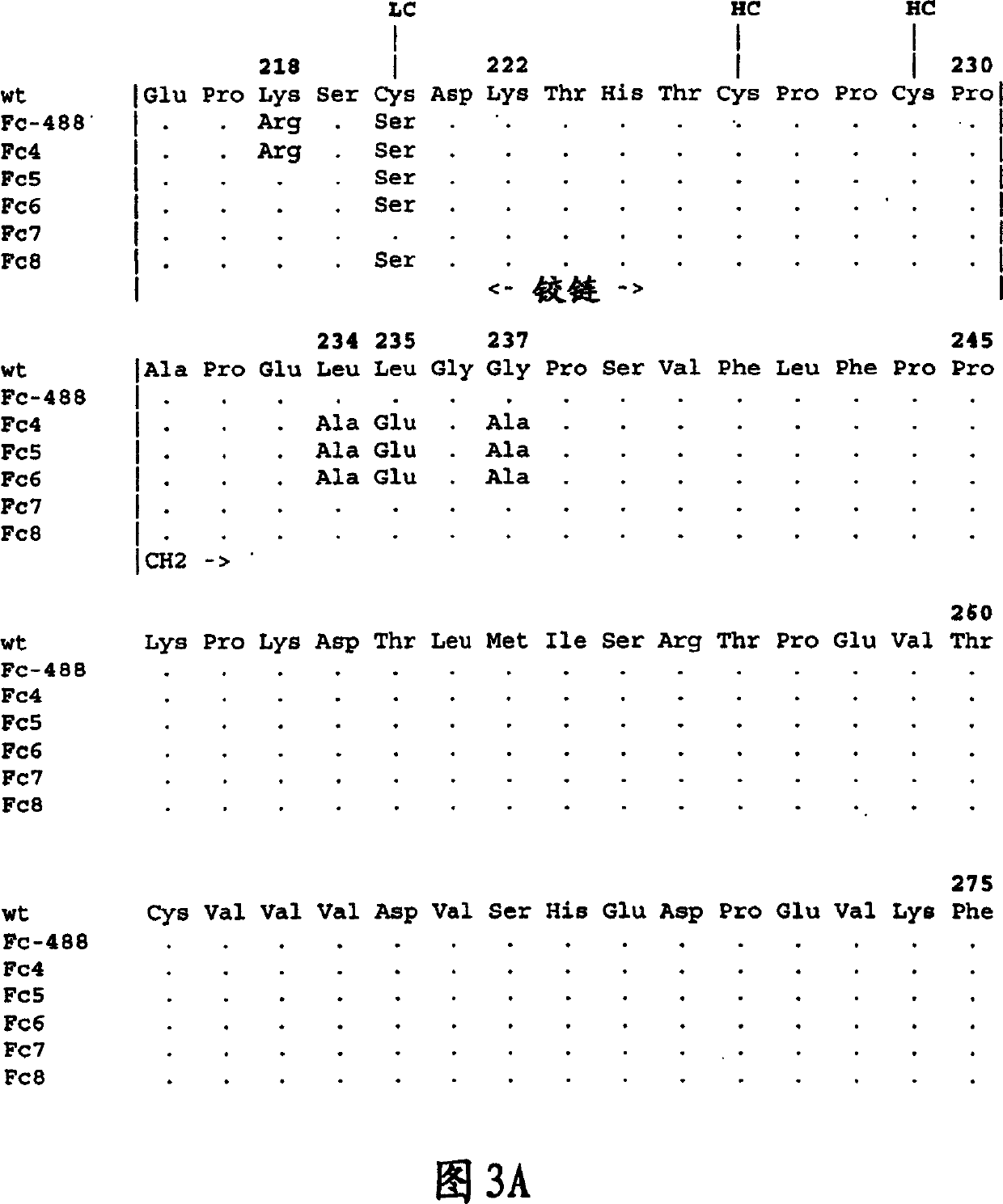
[0302] Structural analysis of TACI-Fc protein
[0303] In some cases, TACI-Fc fusion proteins were partially purified prior to analysis. The conditioned medium of Chinese hamster ovary cells was sterile filtered through a 0.22 μm filter, and the TACI-Fc protein was captured on a protein A column. Material bound to protein A was eluted and passed through an S-200 size exclusion column for final purification.
[0304] Western blot analysis was performed on both the conditioned medium of the cells and the purified protein to assess the structural stability of the TACI-Fc protein. Briefly, samples of protein or supernatant were transferred to nitrocellulose membranes and treated with peroxidase-conjugated goat anti-mouse IgG2a (Boehringer Mannheim), or peroxidase-conjugated goat anti-human IgG Fc TACI-Fc protein was detected with specific antiserum (Pierce).
[0305] Amino acid sequence analysis of the amino terminus was performed on the Model 476A and 494 protein ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com