N-(2,6-dibenzyloxy benzoyl)-N'-substituted urea compound and preparation method and application thereof
A kind of technology of dibenzyloxybenzoyl group and compound, applied in N--N'-substituted urea compound and preparation field thereof, can solve the problems such as no bibliographical report etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
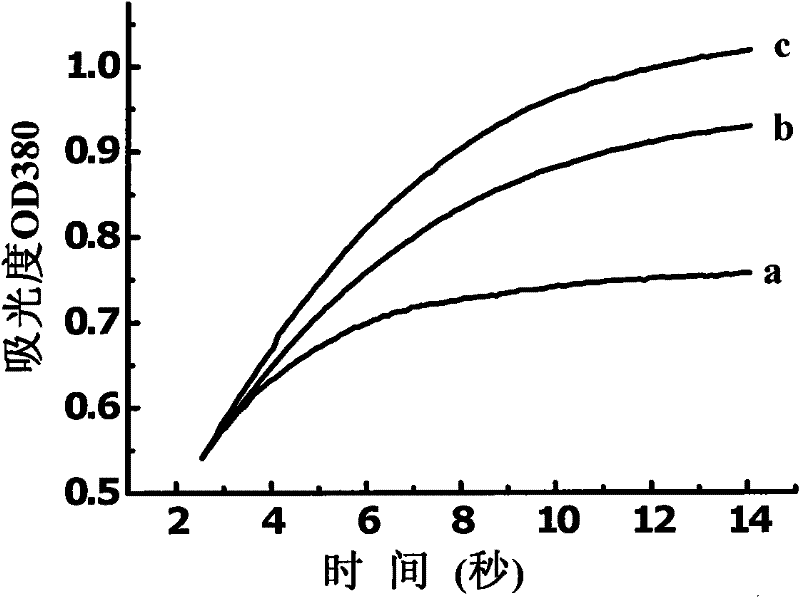
Image
Examples
preparation example Construction
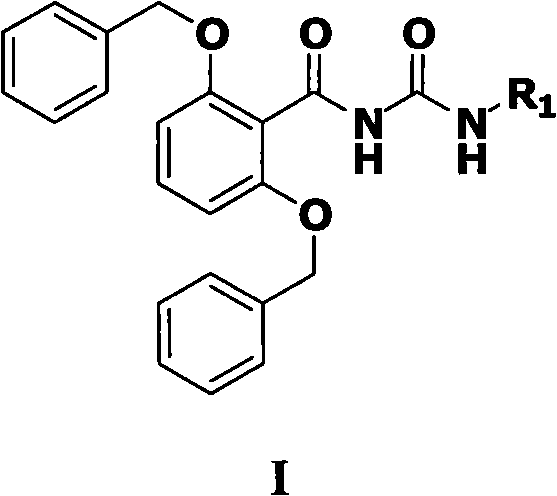
[0039] A method for preparing compounds shown in formula I provided by the present invention, which comprises the following steps:
[0040] 1) Dissolve sodium benzyl alkoxide and 2,6-difluorobenzonitrile in dimethyl sulfoxide, heat up to 110°C-130°C, stir for 5-10 hours, cool to room temperature, add ice water to the reaction solution, After stirring for 30 minutes, a large amount of solid precipitated out, which was suction filtered, washed with water, and dried to obtain a white solid, which was recrystallized to obtain a white flaky crystal 2,6-dibenzyloxybenzonitrile (abbreviation: compound VIII);
[0041] 2) Dissolve compound VIII obtained in step 1) in benzyl alcohol, add potassium hydroxide and a small amount of water, stir at 120°C to 140°C for 10 hours to 15 hours, distill off the solvent under reduced pressure, and add ice to the residue water, stirred for 30 minutes, a large amount of solids were precipitated, suction filtered, washed with water, and dried to obtain...
Embodiment 1
[0048] Preparation of 2,6-dibenzyloxybenzonitrile
[0049]
[0050] Dissolve 0.5 g of sodium benzyl alcohol in 10 ml of dimethyl sulfoxide, and add 2,6-difluorobenzonitrile under stirring. After rapidly raising the temperature to 115°C, stirring was continued for 8 hours. After cooling to room temperature, 100 ml of ice water was poured into the reaction liquid, and stirred for 30 minutes. A large amount of solid precipitated, which was suction filtered, washed with water, and dried to obtain a white solid. Recrystallized from ethanol to obtain compound VIII in the form of white flaky crystals.
[0051] 1 H-NMR (400Hz, DMSO-d 6 )δ: 5.19 (4H, s, -CH 2 ), 6.60 (2H, d, Ar-H), 7.30-7.35 (3H, m, Ar-H), 7.38 (4H, t), 7.45 (4H, d).
Embodiment 2
[0053] Preparation of 2,6-dibenzyloxybenzamide
[0054]
[0055] Dissolve 0.4 g of 2,6-dibenzylbenzonitrile (VIII) in 7 ml of benzyl alcohol, add 0.6 g of KOH and 0.1 ml of water, and stir at 130° C. for 12 hours. The solvent was distilled off under reduced pressure, and 100 ml of ice water was poured into the reaction liquid, stirred for 30 minutes, a large amount of solid precipitated out, filtered with suction, washed with water, and dried to obtain a white solid. Silica gel column chromatography (petroleum ether / ethyl acetate=12 / 1 (v / v)) gave Compound IX as a white powdery solid.
[0056] 1 H-NMR (400Hz, DMSO-d 6 )δ: 5.15 (4H, s, -CH 2 ), 6.60 (2H, d, Ar-H), 7.18-7.48 (12H, m, Ar-H); EI-MS m / z 333.2 (M + ), 91.1 (100%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com