Skin-permeable selective cyclooxygenase-2 inhibitor composition
A technology of composition and monohydric alcohol, which is applied in the direction of active ingredients of heterocyclic compounds, drug combinations, non-central analgesics, etc., can solve the problems such as the difficulty in formulating therapeutic effect COX-2 inhibitory pharmaceutical compositions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
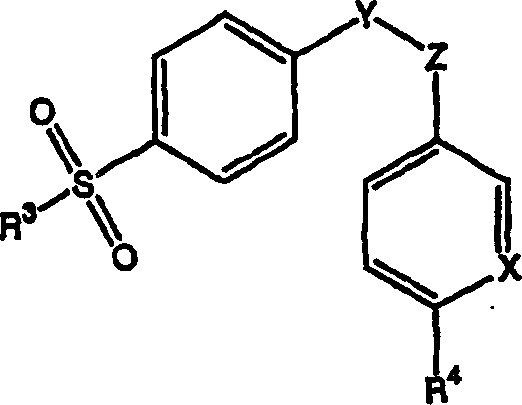
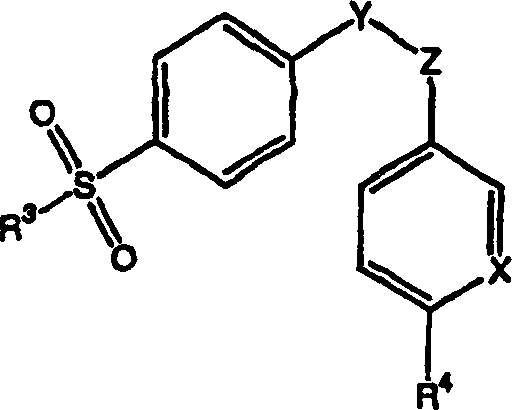
[0067]According to the first embodiment of the present invention, the skin penetration rate of the therapeutic drug of the composition is at least equal to that provided by a reference solution of the therapeutic drug dissolved in 70% ethanol aqueous solution, preferably the rate is not less than about 10 μg / cm 2 ·sky. When skin penetration rates, or ranges for such rates, are specified herein, it is understood that rates are determined by standard testing, for example using human cadaver skin.
[0068] As an example of such a test, a Franz diffuser cell (cadaveric skin film of suitable area, eg, a 20mm diameter disc) and a suitable recipient fluid, described in more detail in the Examples below, may be used. Those skilled in the art are able to select a suitable receptor fluid, but the preferred receptor fluid of the present invention is 1% Tween 80 solution and 6% polyethylene glycol (20) oleyl ether (oleth-20) solution. The receptor fluid is maintained at a suitable temp...
Embodiment 1
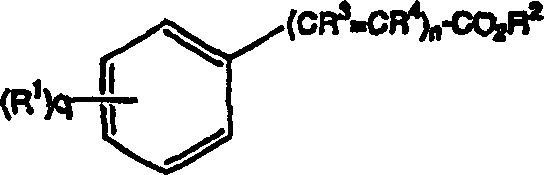
[0156] Saturated solutions of celecoxib were prepared in the following solvents: 70% ethanol (EtOH) in water, ethanol, PEG-400, and propylene glycol (PG). The skin penetration performance of the solutions was tested according to the above method, and each test solution was tested with 250 μl droplets. The results are shown in Table 1.
Embodiment 2
[0158] Saturated solutions of valdecoxib were prepared and tested exactly as described in Example 1 for the celecoxib solution. The results are shown in Table 1.
[0159] drug
Valdecoxib
70%
EtOH
EtOH
PEG-
400
PG
70%
EtOH
EtOH
PEG-
400
PG
concentration
(mg / ml)
15.2
91.4
297
33.3
12.7
7.48
210
23.6
skin flux
(μg / cm 2 ·sky)
15.7
±3.83
5.62
±1.49
ud
ud
12.8±
4.96
1.44±
0.54
ud
ud
[0160] ud = not detected
[0161] No skin penetration of celecoxib and valdecoxib was observed over a 24 hour period when PEG-400 or propylene glycol were used as solvents.
[0162] Surprisingly, 70% ethanol in water provided greater skin flux for both celecoxib and valdecoxib than ethanol alone. With this solvent, t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com