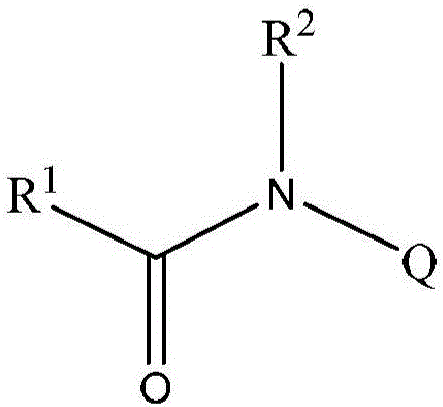
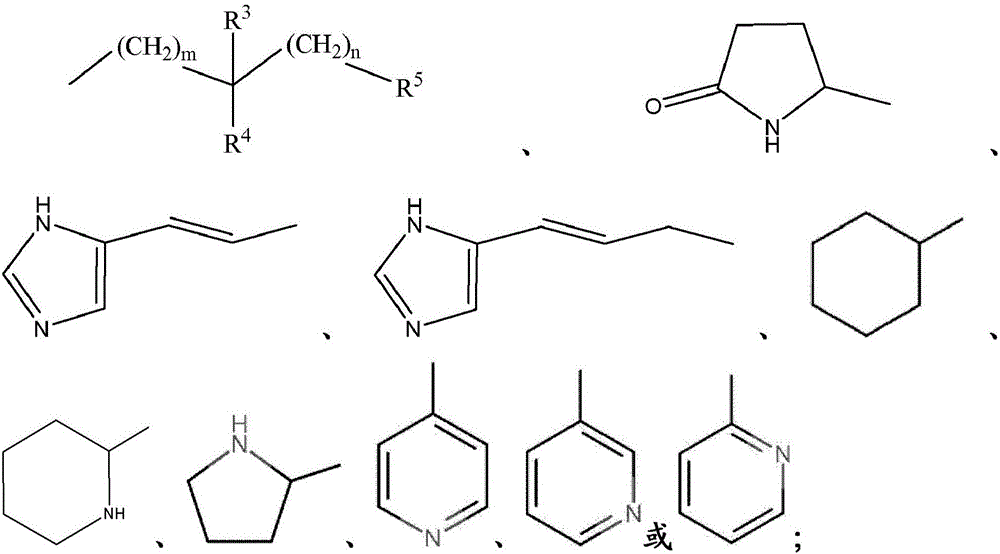
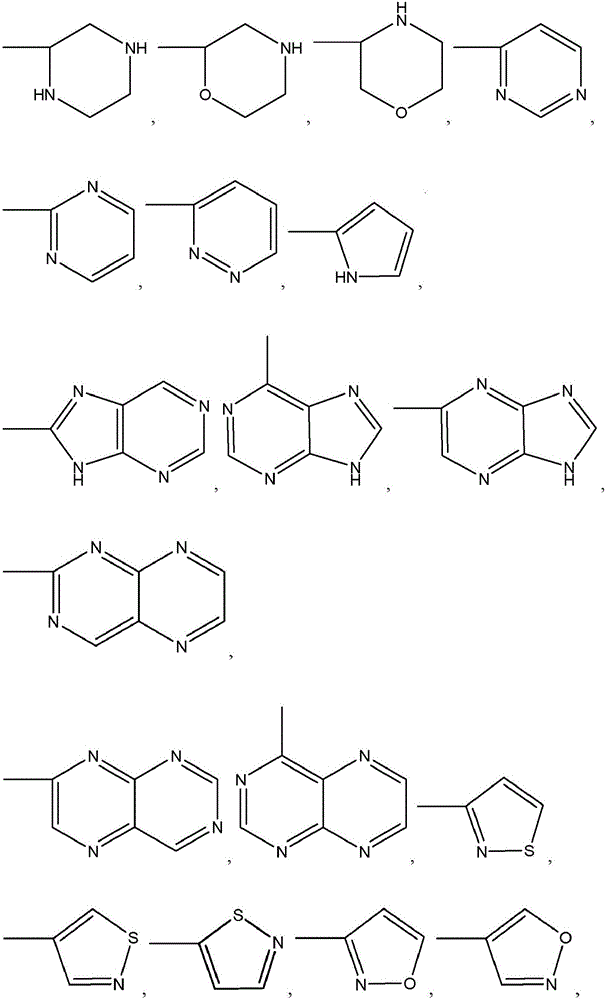
Amide compounds and methods for the production and use thereof
A technology for compounds and medicinal salts, applied in chemical instruments and methods, active ingredients of heterocyclic compounds, drug combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0429] Antiviral activity of compounds of formula I against Coxsackie virus in vitro
[0430] The trypsin-dependent strain HCXV A2, which was pre-adapted and caused the death of coxsackievirus-infected mice, was used in this study.
[0431] The experiment was carried out in mice weighing 6 to 7 g. Animals were infected intramuscularly at a dose of 0.1 mL / mouse. The infectious dose used which resulted in the mortality of mice was 10 LD 50 .
[0432] The ability of a compound to provide a therapeutic effect is determined by the mortality of HCXV A2 virus infected mice in the experimental group relative to the group of untreated animals.
[0433] Study compounds and placebo were administered orally according to the treatment regimen. A saline solution was administered to the mice as a placebo. Untreated animals served as negative controls and were kept in separate rooms under the same conditions as the experimental animals.
[0434] For this experiment, groups were formed...
Embodiment 2
[0442] Antiviral activity of compounds of general formula I against mouse-adapted RS virus
[0443] The antiviral effectiveness of chemical compounds against RSV in an in vitro mouse model was determined against the human virus hRSV, which was previously adapted to grow in mouse lungs. Mice were briefly anesthetized with ether at a volume of 0.05 mL / mouse and treated with 0.5 logTCID 50 doses to infect the virus intranasally. The compound under study was administered orally once daily for 5 days at a dose of 30 mg / kg according to the treatment regimen. The first administration was performed 24 hours after infection. A saline solution was administered to the mice as a placebo. Untreated animals served as negative controls and were kept in separate rooms under the same conditions as the experimental animals. Each experimental group contained 12 animals. Ribavirin was used as reference drug at a dose of 40 mg / kg.
[0444] The antiviral activity of the investigated compoun...
Embodiment 3
[0456] Antiviral activity of compounds of general formula I against RS virus in an immune system suppressed mouse model
[0457] Compounds against human respiratory syncytial virus (strain A2, infection titer is 5×10 6 TCID 50 / mL) was evaluated in the Balb / c mouse model of viral pneumonia. Animals were inoculated intranasally with virus in a volume of 50 μL under brief ether anesthesia. The animals' immune response to RS virus was suppressed by intraperitoneal administration of cyclophosphamide at a dose of 100 mg / kg 5 days before infection. Starting 24 hours after infection, the compound under study was administered once daily for 5 days according to the treatment protocol at a dose of 30 mg / kg. Compound activity was determined by the reduction of pulmonary edema in respiratory syncytial virus-infected lungs relative to controls at 5 days post-infection.
[0458] The results shown in Table 6 for some specific compounds of general formula I (without any limitation to th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com