Preparation process of lavo-ofloxacin and ofloxacin
A technology of ofloxacin and peroxybenzoic acid is applied in chemical instruments and methods, preparation of organic compounds, preparation of cyanide reactions, etc., and can solve the problems of reduced reaction speed, more impurities, and increased side reactions, etc. The effect of improving production level, reducing reaction temperature and reducing side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The preparation of embodiment 1 formula (VIII) compound
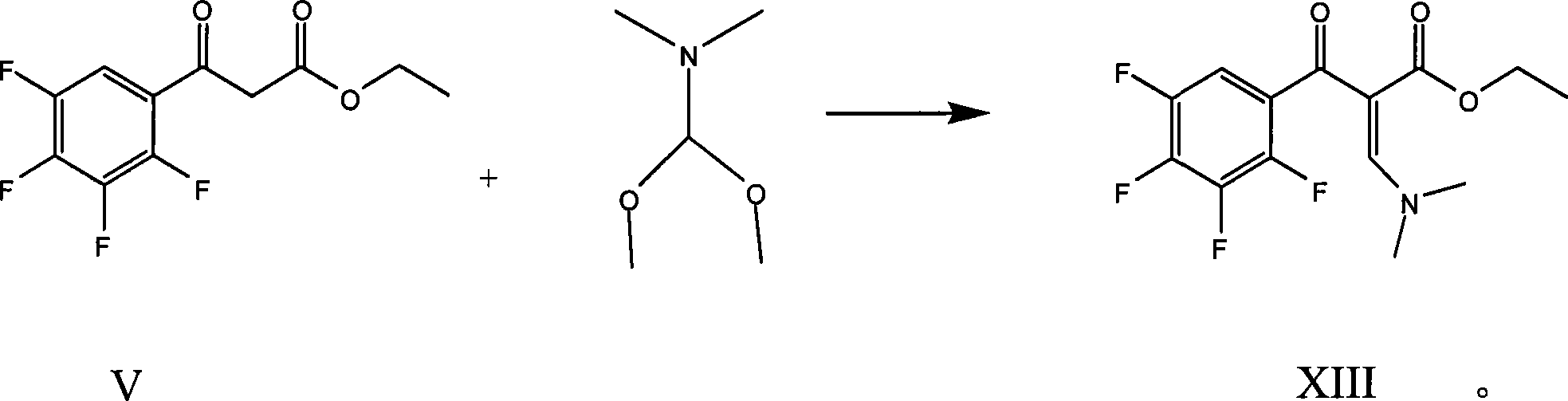
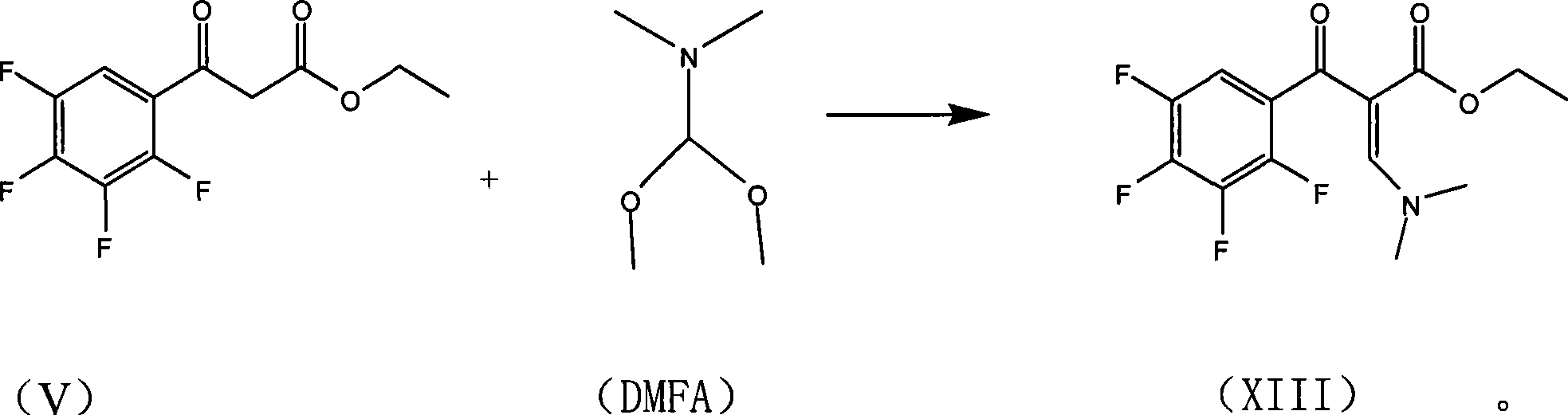
[0042] (1) Preparation of formula (XIII) compound
[0043]
[0044] Put 150ml of toluene, 50g (V), 27g of DMFA, and 1.0g of acetic anhydride into a 500ml three-necked bottle in turn, stir and heat up to 52°C, and keep warm for a while. After incubating at 50-55°C for 90 minutes, add 200ml of water and stir for 3 minutes, then add an appropriate amount of hydrochloric acid to adjust the pH to 5-6. The water layer was separated, and the oil layer was the toluene solution of (XIII) (sampled for liquid phase analysis, the liquid phase content of compound (XIII) was 90%). It can be directly used in the next step without separation.
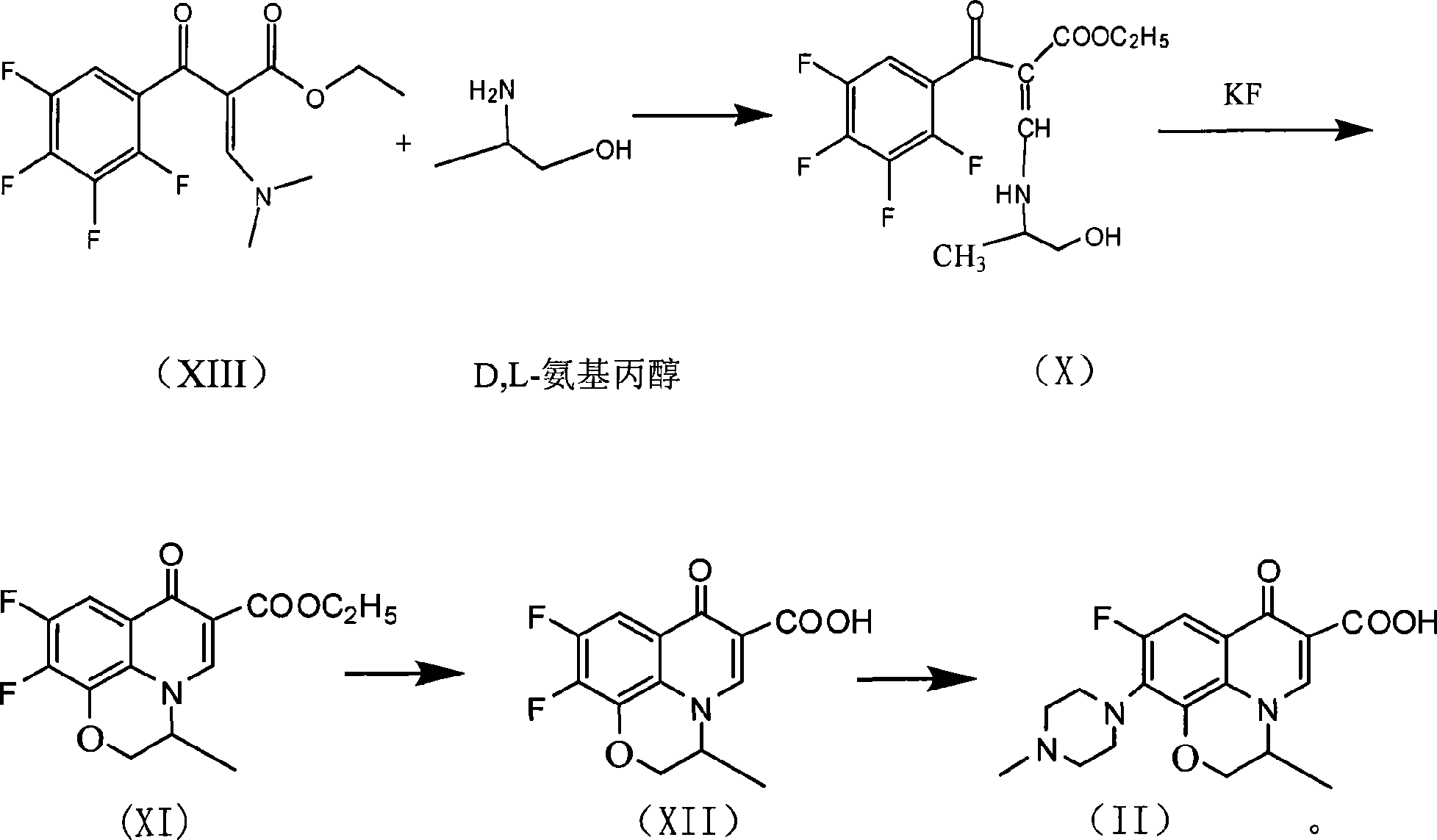
[0045] (2) preparation of formula (VII) compound
[0046]
[0047] The temperature of the toluene solution in the previous step (XIII) was raised to 35°C, and 14 g of L-aminopropanol was added dropwise with stirring, and the drop was completed within 30 minutes. After dropping a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com