Levofloxacin hydrochloride sustained-release eye drops
A technology of levofloxacin hydrochloride and slow-release eye drops, which is applied to medical preparations of non-active ingredients, organic active ingredients, sensory diseases, etc., can solve problems such as damage to the eyes, change of cell permeability, and degeneration of lacrimal gland function. Effects of improving biosafety, wide-ranging antibacterial and sterilizing capabilities, and reducing risk of damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-6
[0015] Embodiment 1-6 prepares the levofloxacin hydrochloride sustained-release eye drops raw material component and dosage without preservative
[0016]
[0017] According to the technical scheme of the present invention, the optional adjuvant species for preparing levofloxacin hydrochloride sustained-release eye drops without preservatives is not limited to the species listed in the above table, and can also have the following multiple options:
[0018] Metal ion complexing agent: metal ion complexing agents commonly used in pharmacy can be used, such as any one of disodium edetate, sodium gluconate, sodium tartrate, sodium citrate, aminotriacetic acid or any of the described varieties Combination; the amount of the metal ion complexing agent is expressed in weight ratio with levofloxacin, levofloxacin hydrochloride: metal ion complexing agent=1:0.01~0.50.
[0019] Use osmotic pressure regulator to adjust the osmolar concentration of finished eye drops to be 280~330mOsmol...
experiment example 1
[0023] Experimental Example 1 Pharmacodynamics Test
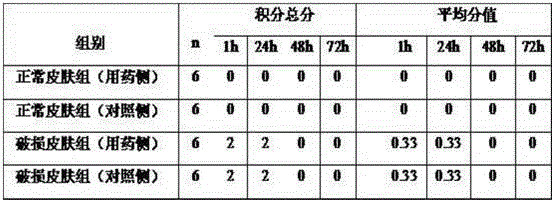
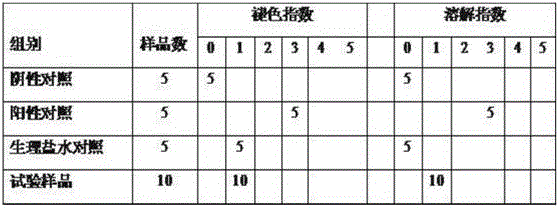
[0024] The preparation prepared by the method described in the embodiment of the present invention 2 is used as test sample, adopts 0.3% levofloxacin hydrochloride eye drops (reference preparation I, Beijing Lixiang Pharmaceutical Co., Ltd., trade name: Langyue, specification 24mg: 8ml), 0.3% levofloxacin hydrochloride eye drops (reference preparation II, Shandong Bausch & Lomb Freda Pharmaceutical Co., Ltd., trade name: Helen, specification 5ml: 15mg), normal saline are as reference substance, pass through pharmacodynamics test, illustrate hydrochloric acid of the present invention Levofloxacin eye drops have a sustained-release effect and can improve the therapeutic effect.
[0025] 1. Establishment of injury-type bacterial conjunctivitis model
[0026] 1.1 Preparation of infection bacteria suspension: Dilute Staphylococcus aureus solution cultured for 2 days to 5×10 with 0.9% sodium chloride injection 9 cfu ml -1 (Det...
experiment example 2
[0040] Pharmacokinetic experiment in experimental example 2 in rabbit eye aqueous humor
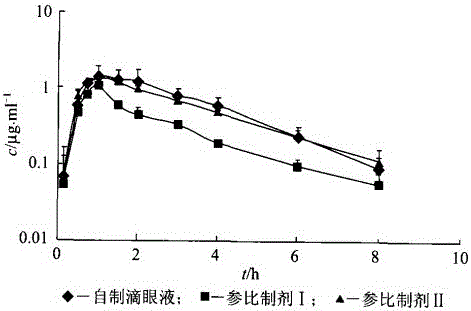
[0041]The preparation prepared by the method described in the embodiment of the present invention 2 is used as test sample, adopts 0.3% levofloxacin hydrochloride eye drops (reference preparation I, Beijing Lixiang Pharmaceutical Co., Ltd., trade name: Langyue, specification 24mg: 8ml), 0.3% levofloxacin hydrochloride eye drops (reference preparation II, Shandong Bausch & Lomb Freda Pharmaceutical Co., Ltd., trade name: Helen, specification 5ml: 15mg), normal saline as reference substance, through the pharmacokinetics in the aqueous humor of rabbit eyes The scientific experiment shows that the levofloxacin hydrochloride eye drops of the present invention has sustained release effect and can improve the bioavailability of medicine.
[0042] 1. Administration method: Self-control, cross-over experimental design is adopted, and the washout period is 7 days. Twenty-five healthy New Zealand r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com