Synthesis method of gatifloxacin cyclic ester
A gatifloxacin cyclization ester and synthesis method technology, applied in the direction of organic chemistry, can solve the problems of long synthesis route steps, lower raw material utilization rate, poor atom economy, etc., achieve convenient post-processing and reduce tar-like substances The effect of production and short synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
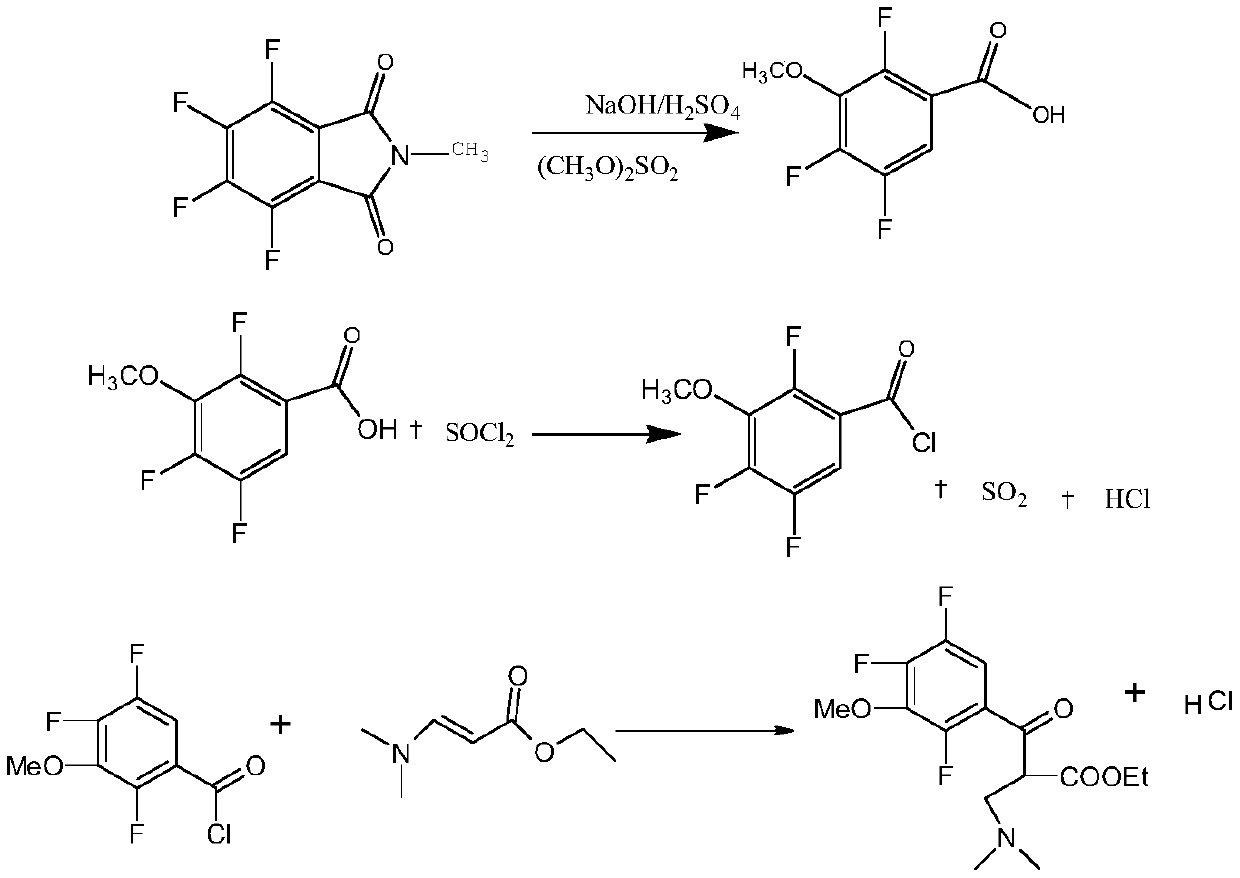
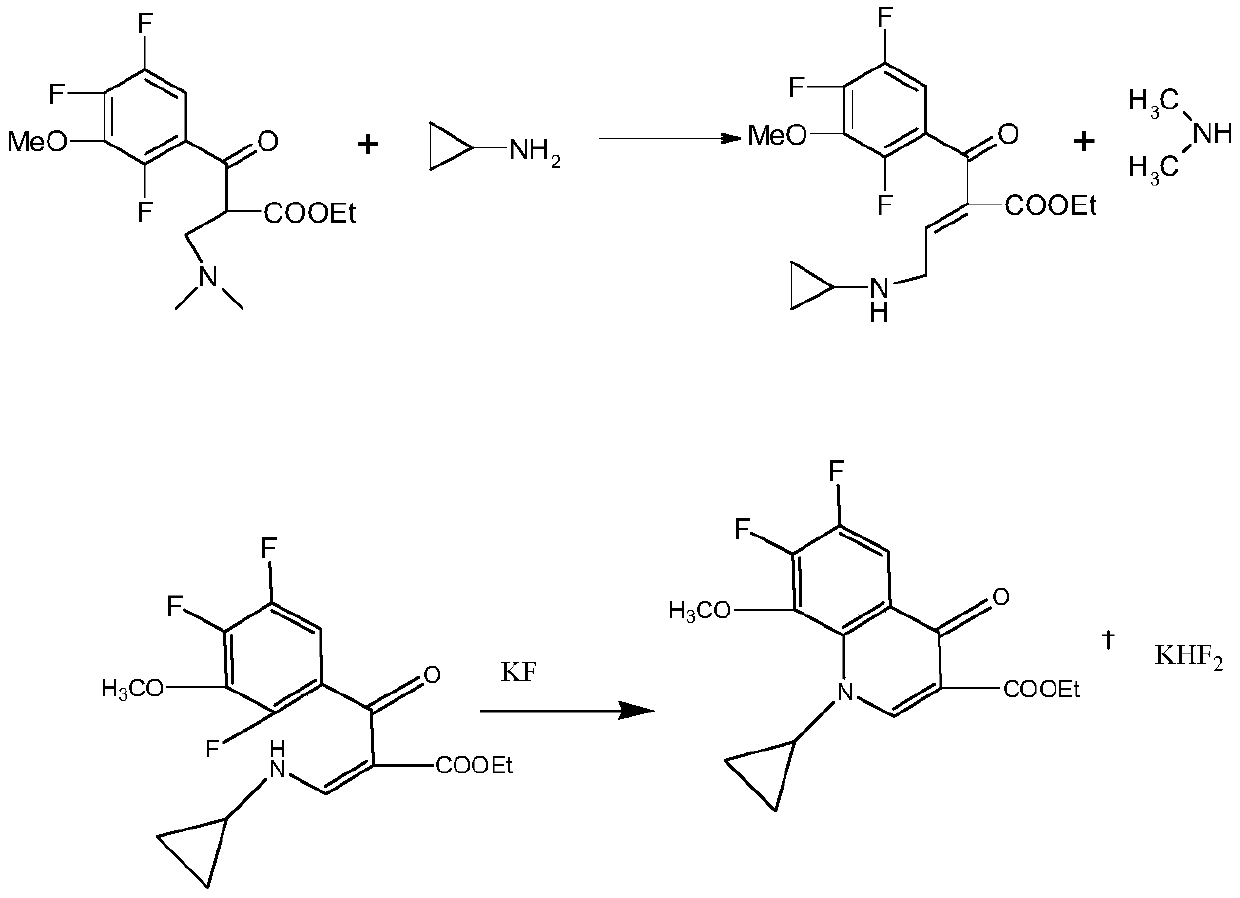
[0040] A kind of synthetic method of gatifloxacin cyclic ester, comprising:
[0041] (1) Add 100mL of 30% sodium hydroxide solution to a 500mL reactor, and mix 100g of 3,4,5,6-tetrafluoro-N-methylphthalimide and sodium hydroxide evenly , heated and refluxed for 10 hours, and reacted to obtain 2,4,5-trifluoro-3-hydroxyphthalate sodium; then the temperature was controlled below 70°C, Add sulfuric acid to sodium diformate, carry out decarboxylation reaction and acidify to pH 3.05; after that, adjust the temperature to 105°C, keep the temperature for 8 hours, and cool down to 25°C to obtain 2,4,5-trifluoro-3-hydroxybenzoic acid ;
[0042](2) Transfer the obtained 2,4,5-trifluoro-3-hydroxybenzoic acid into the methylation device, add alkali to adjust the pH to 9.07, control the temperature to be less than 35°C, add dimethyl sulfate, and at the same time, pass Add lye to control the pH to 8.55. After the addition of dimethyl sulfate, raise the temperature to 80°C, adjust the pH to...
Embodiment 2
[0046] A kind of synthetic method of gatifloxacin cyclic ester, comprising:
[0047] (1) Add 100mL of 30% sodium hydroxide solution to a 500mL reactor, and mix 100g of 3,4,5,6-tetrafluoro-N-methylphthalimide and sodium hydroxide evenly , heated up and refluxed for 8 hours, and reacted to obtain 2,4,5-trifluoro-3-hydroxyphthalate sodium; then the temperature was controlled below 70°C, Add sulfuric acid or hydrochloric acid to sodium diformate for decarboxylation and acidification to pH 0.87; after that, adjust the temperature to 100°C, keep the temperature for 6 hours, and cool down to 20°C to obtain 2,4,5-trifluoro-3-hydroxyl benzoic acid;
[0048] (2) Transfer the obtained 2,4,5-trifluoro-3-hydroxybenzoic acid into the methylation device, add alkali to adjust the pH to 9.49, control the temperature to be less than 35°C, add dimethyl sulfate, and simultaneously add Control the pH of the lye to 8.51. After the addition of dimethyl sulfate, raise the temperature to 75°C, adjus...
Embodiment 3
[0052] A kind of synthetic method of gatifloxacin cyclic ester, comprising:
[0053] (1) Add 100mL of 30% sodium hydroxide solution to a 500mL reactor, and mix 100g of 3,4,5,6-tetrafluoro-N-methylphthalimide and sodium hydroxide evenly , heated up and refluxed for 12 hours, and reacted to obtain 2,4,5-trifluoro-3-hydroxyphthalate sodium; then the temperature was controlled below 70°C, Add sulfuric acid or hydrochloric acid to sodium diformate, decarboxylate and acidify to pH 3.98; after that, adjust the temperature to 110°C, keep the temperature for 10 hours, and cool down to 30°C to obtain 2,4,5-trifluoro-3-hydroxyl benzoic acid;
[0054] (2) Transfer the obtained 2,4,5-trifluoro-3-hydroxybenzoic acid into the methylation device, add alkali to adjust the pH to 9.53, control the temperature to be less than 35°C, add dimethyl sulfate, and simultaneously add Control the pH of the lye to 9.47. After the addition of dimethyl sulfate, raise the temperature to 85°C, adjust the pH ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com