Novel crystalline forms of gatifloxacin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Form A
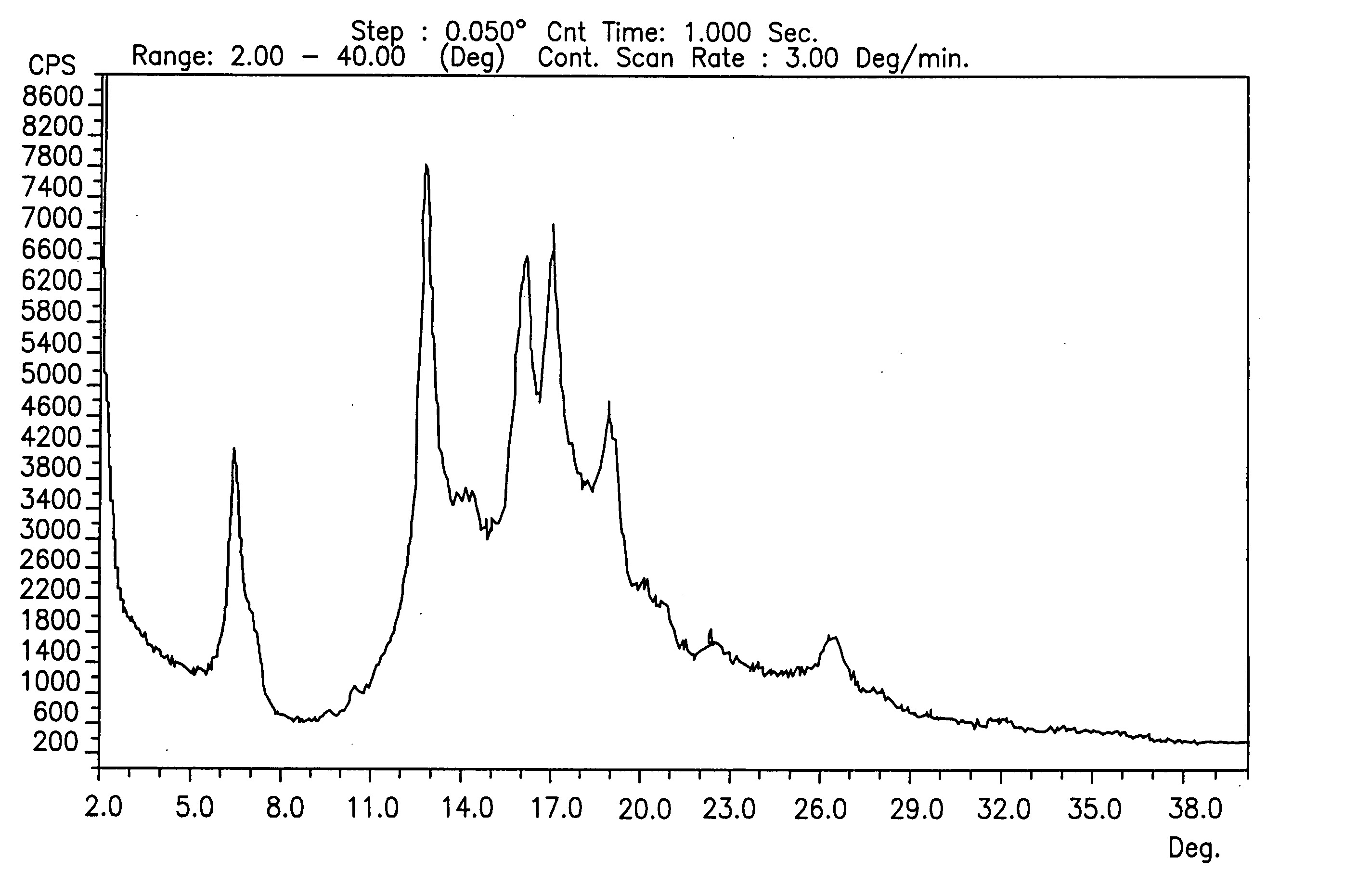
[0233] 3 g of gatifloxacin were slurried in 20 mL of iso-propanol (IPA). The mixture was slurried at ambient temperature for a slurry time of 24 hours with a magnetic stirrer. The mixture was filtered under vacuum, rinsed with iso-propanol (IPA) (10 mL) and analyzed by XRD analysis and showed to be form A.
example 2
Form B
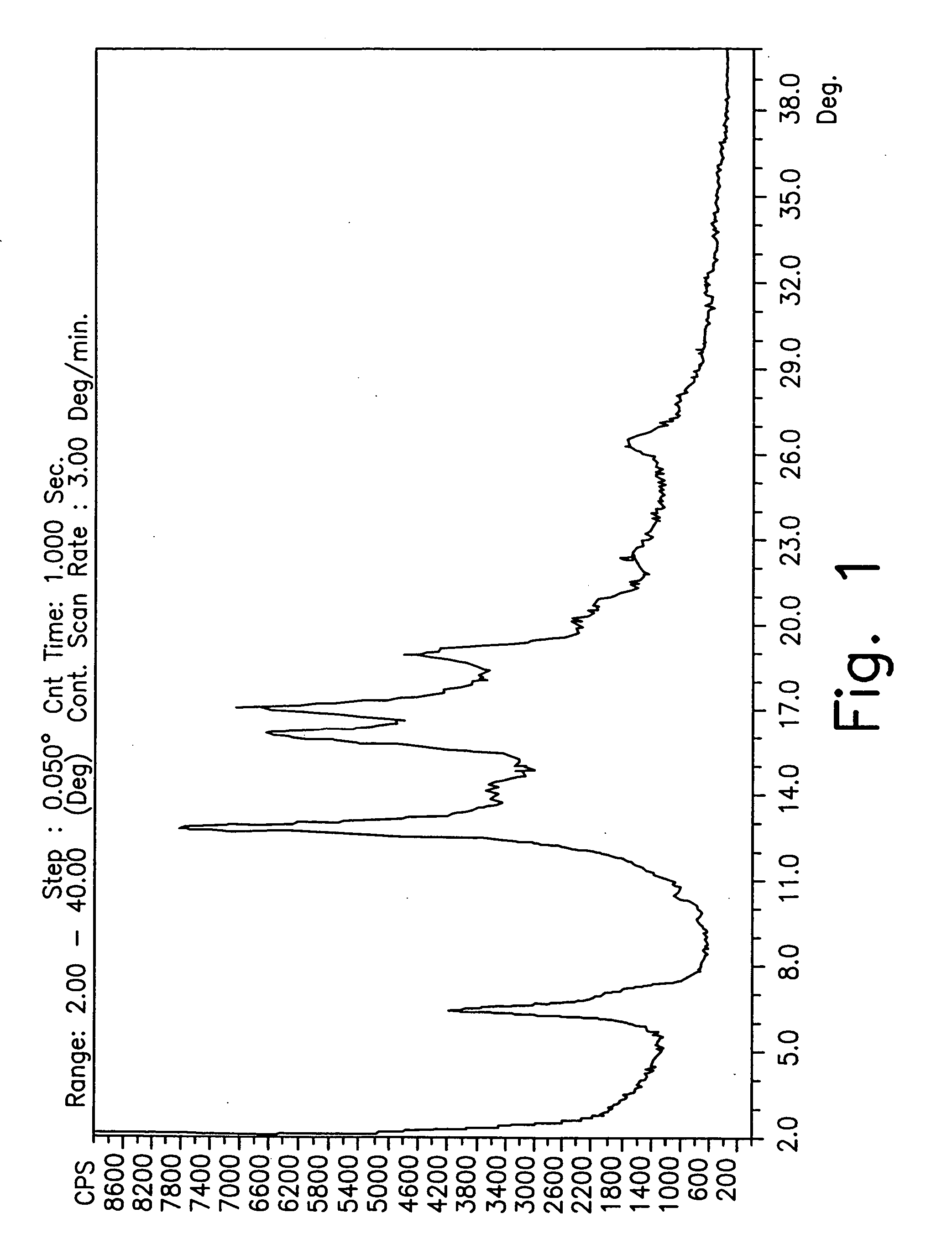
[0234] 3 g of gatifloxacin were slurried in 20 mL of 1-butanol. The mixture was stirred at ambient temperature for a slurry time of 24 hours with a magnetic stirrer. Then the mixture was filtered under vacuum, the isolated solid rinsed with 1-butanol (10 mL), and analyzed by XRD analysis.
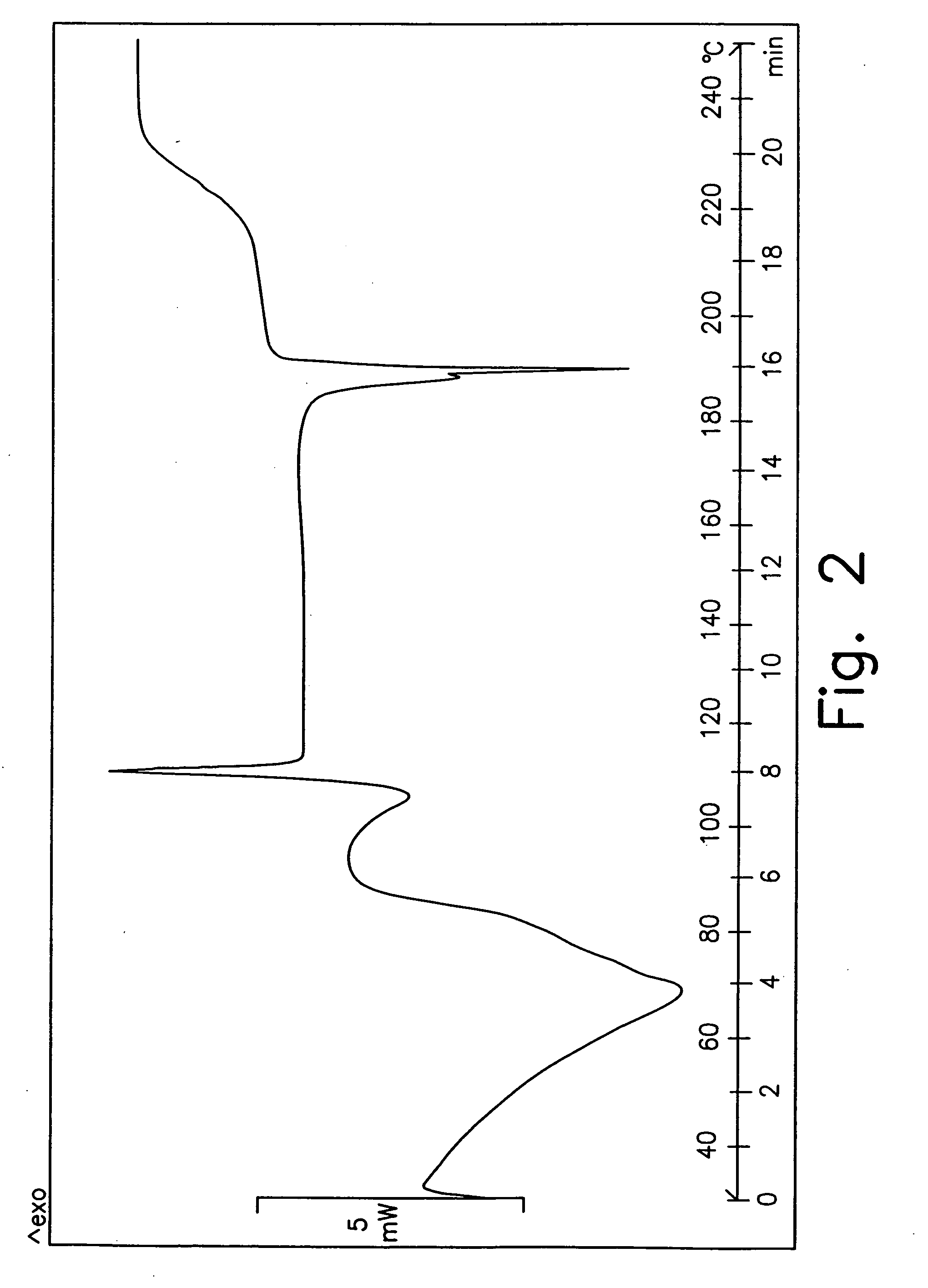
[0235] A second portion of the solid obtained after filtration was dried under vacuum at 50° C. for 24 hours. This resulted in a partially amorphous form B.
example 3
Form B
[0236] 3 g of gatifloxacin were slurried in 20 mL of EtOH absolute. The mixture was stirred at ambient temperature for a slurry time of 24 hours with a magnetic stirrer. Then the mixture was filtered under vacuum, the isolated solid rinsed with absolute EtOH (10 mL), and analyzed by XRD. The product was partially amorphous form B.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com