Preparation method of moxifloxacin hydrochloride and intermediate of moxifloxacin hydrochloride
A technology for moxifloxacin hydrochloride and moxifloxacin ethyl ester is applied in the field of preparation of moxifloxacin hydrochloride and its intermediates, which can solve the problems of many synthesis steps, low product purity, narrow supply of raw materials and the like, and achieves high atom economy. , environmental friendliness, raw material price and cost reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
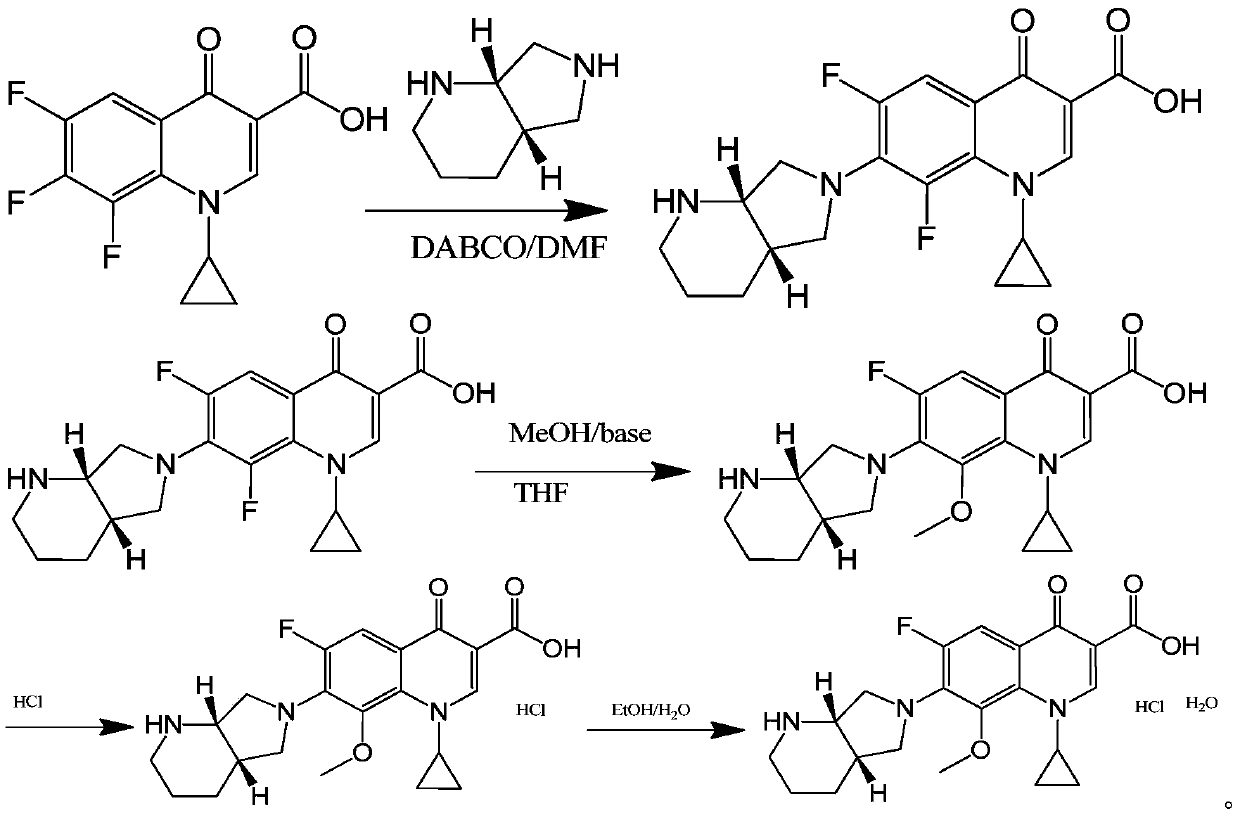
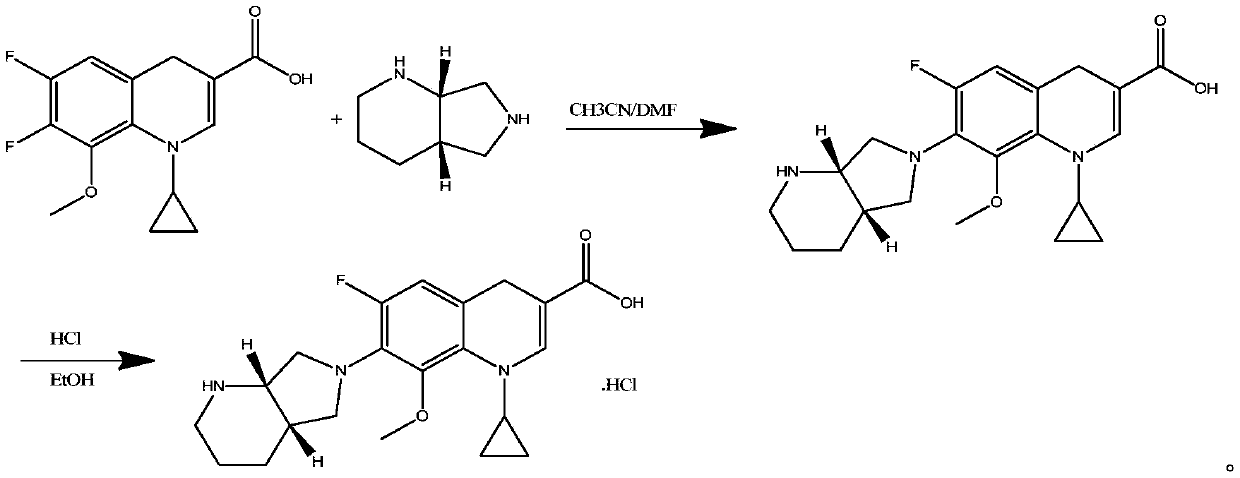
Image
Examples
Embodiment 1
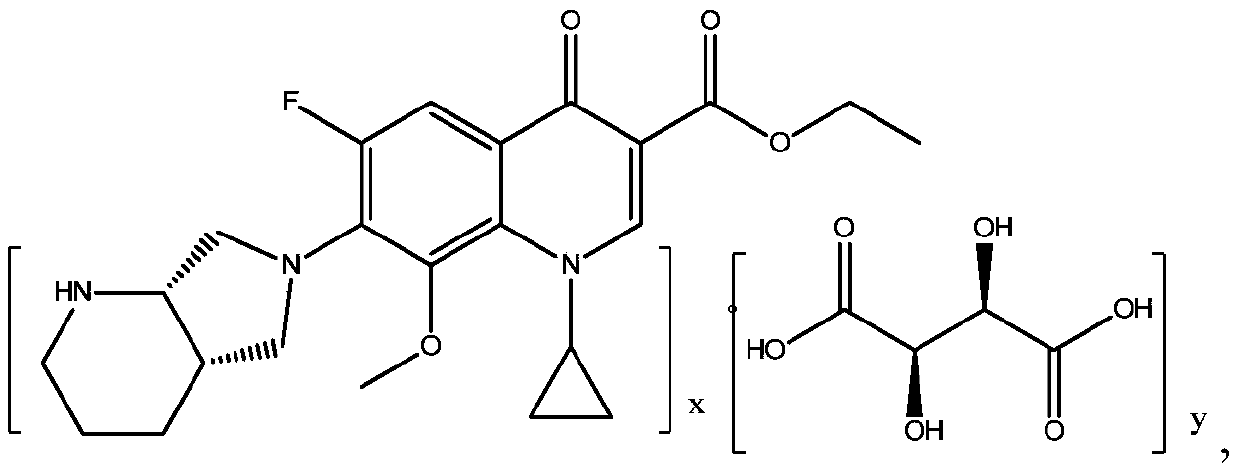
[0044] Embodiment 1: the preparation of moxifloxacin ethyl ester tartrate
[0045] In a 2L four-neck flask, add 100g (0.309mol) of ticycline ester, 40.5g (0.321mol) of (S,S)-2,8-diazabicyclo[4,3,0]nonane, acetonitrile 1000ml, stirred, put in 32.5g (0.321mol) of triethylamine, AlCl 3 2.05g (0.015mol), heat up to 60°C, keep warm for about 3 hours, the system is basically clear, take a sample, TLC (developing agent is methanol: acetone: ammonia water = 15:5:3) detection and tracking basically no reaction raw material (gaticycline ester) spots, cooled to room temperature, filtered, the filtrate continued to heat up to 50°C, added 27.84g (0.185mol) of L-(+)-tartaric acid, stirred and kept for about 2h, the system precipitated in large quantities, cooled to 0-5°C, filtered After the filter cake was washed with acetonitrile slurry, the filter cake was collected, vacuum-dried to constant weight at 50-80° C., and powdered to obtain 142 g of moxifloxacin ethyl ester tartrate, with a mo...
Embodiment 2
[0046] Embodiment 2: the preparation of moxifloxacin ethyl ester tartrate
[0047] In a 2L four-necked flask, add 100g (0.309mol) of ticycline ester, 40.8g (0.323mol) of (S,S)-2,8-diazabicyclo[4,3,0]nonane, and 1000ml of DMF , stirred, put in DBU50.2g (0.323mol), AlCl 3 2.05g, heat up to 55°C, keep warm for about 3 hours, the system is basically clear, take a sample, TLC (eluent: methanol: acetone: ammonia water = 15:5:3) detect and track basically no reaction raw material (gaticyclate) spots , add 28.1g (0.187mol) of L-(+)-tartaric acid, stir and keep warm for about 2 hours, the system precipitates in large quantities, cool down to 0-5°C, filter, wash the filter cake with cooled DMF and collect the filter cake, 50-80°C Under vacuum drying to constant weight, collect powder, obtain moxifloxacin ethyl ester tartrate 140.7g, molar yield 90.2% (according to moxifloxacin ethyl ester and tartaric acid salify ratio is 2: 1 calculation), related substance purity 99.85% , Optical is...
Embodiment 3
[0048] Embodiment 3: the preparation of moxifloxacin ethyl ester tartrate
[0049] In a 2L four-necked flask, add 100g of ticycline ester, 41.0g (0.325mol) of (S,S)-2,8-diazabicyclo[4,3,0]nonane, 1000ml of DMF, stir, and put Triethylamine 32.9g (0.325mol), ZnCl 2 4.2g (0.031mol), heat up to 75°C, keep warm for about 3 hours, the system is basically clear, take a sample, TLC (developing agent is methanol: acetone: ammonia water = 15:5:3) detection and tracking basically no reaction raw material (gaticycline Ester) spots, cooled to room temperature, filtered, the filtrate continued to heat up to 60°C, added 50.4g (0.335mol) of L-(+)-tartaric acid, stirred and kept for about 2h, the system precipitated in large quantities, cooled to 0-5°C, filtered, After the filter cake is washed with cooled DMF slurry, the filter cake is collected, vacuum-dried to constant weight at 50-80° C., and powdered to obtain 158.8 g of moxifloxacin ethyl ester tartrate, with a molar yield of 88.6% (by ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com