Gatifloxacin dispersible tablets and preparation method thereof
A technology for gatifloxacin and dispersible tablets, applied in the field of gatifloxacin dispersible tablets and their preparation, can solve the problems of large volume and troublesome gatifloxacin tablets, and achieve good fluidity, compressibility, and disintegration. good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] (a) Weigh raw materials and auxiliary materials according to the following weight ratios:
[0020] 200g of gatifloxacin, 40g of crospovidone, 140g of microcrystalline cellulose, 30g of hydroxypropyl cellulose, 7g of stevioside, 4.5g of magnesium stearate, an appropriate amount of 4% povidone K30 ethanol solution;
[0021] (b) Get the gatifloxacin, hydroxypropyl cellulose, microcrystalline cellulose, stevioside and crospovidone of the above-mentioned weight portion ratio, pass through 80 mesh sieves respectively, and cross 60 mesh sieves to mix evenly, Use 4% povidone K30 ethanol solution to make soft material, pass through a 30 mesh sieve to granulate, blow dry at 55°C, and then pass the dry granules through a 24 mesh sieve for granulation, then add the prescribed amount of crospovidone and hard Magnesium fatty acid is mixed and compressed into tablets to make dispersible tablets containing 200 mg gatifloxacin in each tablet.
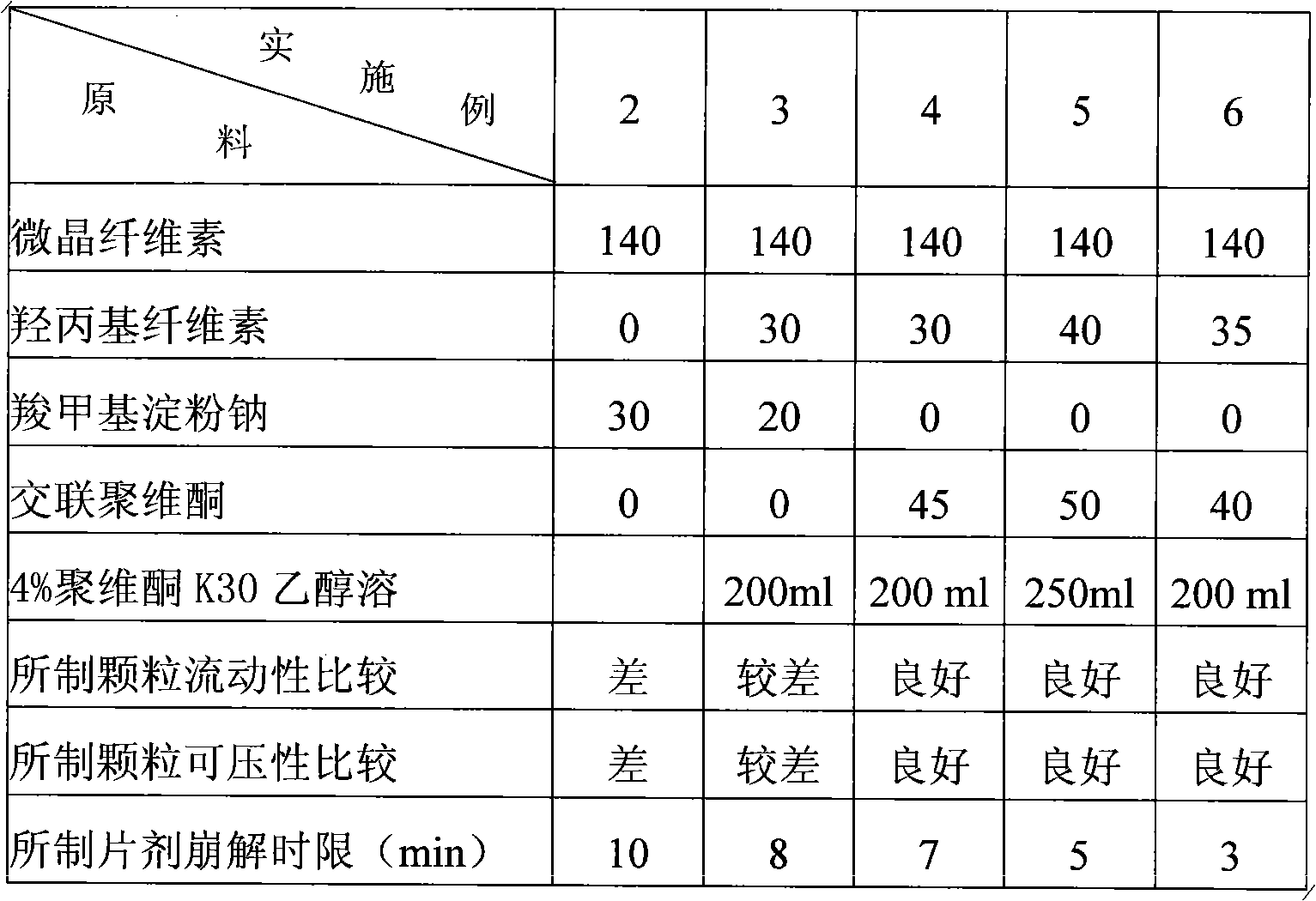
Embodiment 2~6
[0023] (a) Weigh raw materials and auxiliary materials according to the following weight ratios:
[0024] Gatifloxacin 200g, stevioside 7g, magnesium stearate 4.5g, 4% povidone K30 ethanol solution in an appropriate amount; the disintegrants are shown in Table 1.
[0025] (b) Pass gatifloxacin, hydroxypropyl cellulose, microcrystalline cellulose, stevioside and crospovidone respectively through a 80-mesh sieve, then pass through a 60-mesh sieve, mix evenly, and use 4% povidone Ketone K30 ethanol solution is made into soft material, passed through a 30-mesh sieve to granulate, and air-dried at 60°C. The dried granules are then passed through a 24-mesh sieve for sizing, and then the prescribed amount of crospovidone and magnesium stearate are added. After mixing, compress into tablets to make dispersible tablets containing 400 mg of gatifloxacin in each tablet.
[0026] Table 1 The selection of disintegrants in different embodiments (mg)
[0027]
[0028] (c) Get the gatifl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com