Capsules of gatifloxacin, and technique of dry process
A technology of gatifloxacin and preparation process, which is applied in the directions of capsule delivery, antibacterial drugs, etc., can solve the problems of inconvenient operation, loss, slow disintegration, etc., and achieve the effect of simple process and improved product yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
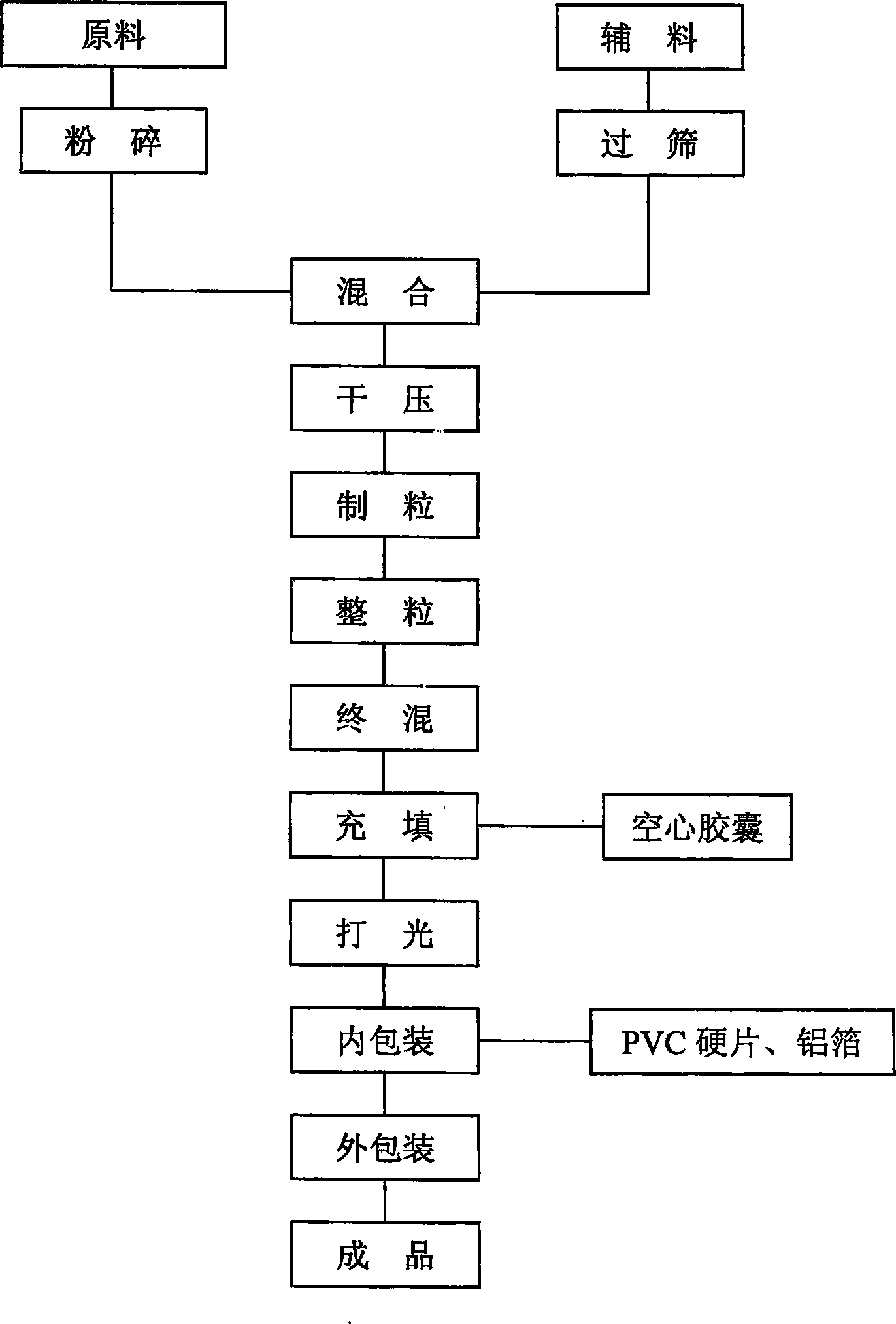
[0014] The present invention will be further described below in conjunction with accompanying drawing:
[0015] Calculated on the basis of 1,000,000 capsules:
[0016] Gatiza 20kg
[0017] Filler 221kg
[0018] Carboxymethyl starch sodium 5kg
[0019] Magnesium stearate 0.5kg
[0020] Fillers include: calcium hydrogen phosphate 70kg, microcrystalline cellulose 75kg, lactose 76kg disintegrant is sodium carboxymethyl starch, lubricant is magnesium stearate
[0021] With reference to the accompanying drawings, according to this process, at first 70kg of calcium hydrogen phosphate, 75kg of microcrystalline cellulose, and 76kg of lactose are used as fillers, 20kg of gatifloxacin, and 5kg of disintegrating agent are mixed uniformly by a fluidized bed one-step granulator, and then dried Press to granulate and pass through a 24-mesh sieve, add 0.5kg of lubricant and mix evenly in a mixer, measure the moisture content to reach the standar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com