Ophthalmic composition as well as preparation method and application thereof
An ophthalmic composition and ocular technology, applied in the field of medicine, can solve problems such as conjunctival hyperemia and edema, affect patients' medication compliance, and prolong patients' recovery time, so as to increase medication compliance, reduce eye irritation, improve The effect of the treatment effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
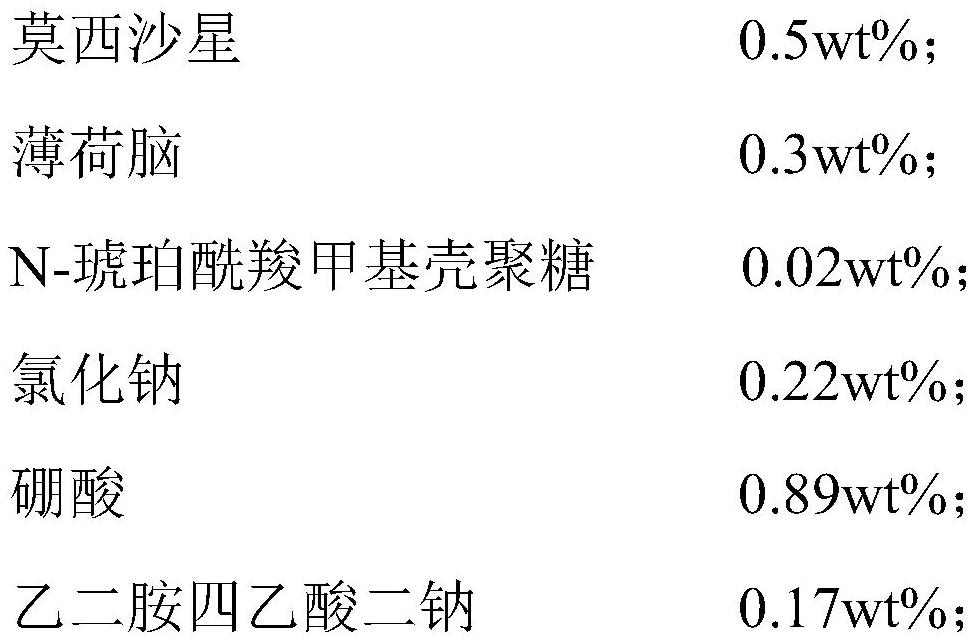
[0036] Add and prepare a total amount of 50wt% water for injection into the preparation tank, and start stirring. At a temperature of 70°C, add moxifloxacin, menthol, N-succinyl carboxymethyl chitosan, and surfactant (polysorbate 80) into the preparation tank and stir to dissolve evenly, then pour into the preparation tank Add prescription amount of osmotic pressure regulator (sodium chloride), pH regulator (boric acid), metal ion chelating agent (ethylenediaminetetraacetic acid disodium) and preservative (benzalkonium chloride), stir and dissolve evenly, and then Add water for injection to make up to full volume, then filter through a 0.22 μm filter membrane, fill it, and prepare an ophthalmic composition with the following composition based on the total mass of the ophthalmic composition:
[0037]
[0038]
[0039] The mass ratio of moxifloxacin, menthol, and N-succinylcarboxymethyl chitosan is 1:0.6:0.04.
Embodiment 2
[0041] The preparation method of the ophthalmic composition of the present embodiment is the same as that of Example 1, except that N-succinylcarboxymethyl chitosan is not added, the osmotic pressure regulator is potassium chloride, and the pH regulator is dihydrogen phosphate Sodium, the metal ion chelating agent are dicalcium edetate, the surfactant is tyloxapol and the preservative is benzalkonium bromide, and the ophthalmic composition of the following composition is obtained in terms of the total mass of the ophthalmic composition:
[0042]
[0043] The mass ratio of moxifloxacin to menthol is 1:0.58.
Embodiment 3
[0045] The preparation method of the ophthalmic composition of the present embodiment is the same as that of Example 1, except that menthol is not added, the osmotic pressure regulator is glycerin, the metal ion chelating agent is citric acid, the surfactant is polyethylene glycol and antiseptic The agent is methylparaben, and the ophthalmic composition of the following composition is obtained in terms of the total mass of the ophthalmic composition:
[0046]
[0047] The mass ratio of moxifloxacin to N-succinylcarboxymethyl chitosan is 1:0.025.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com