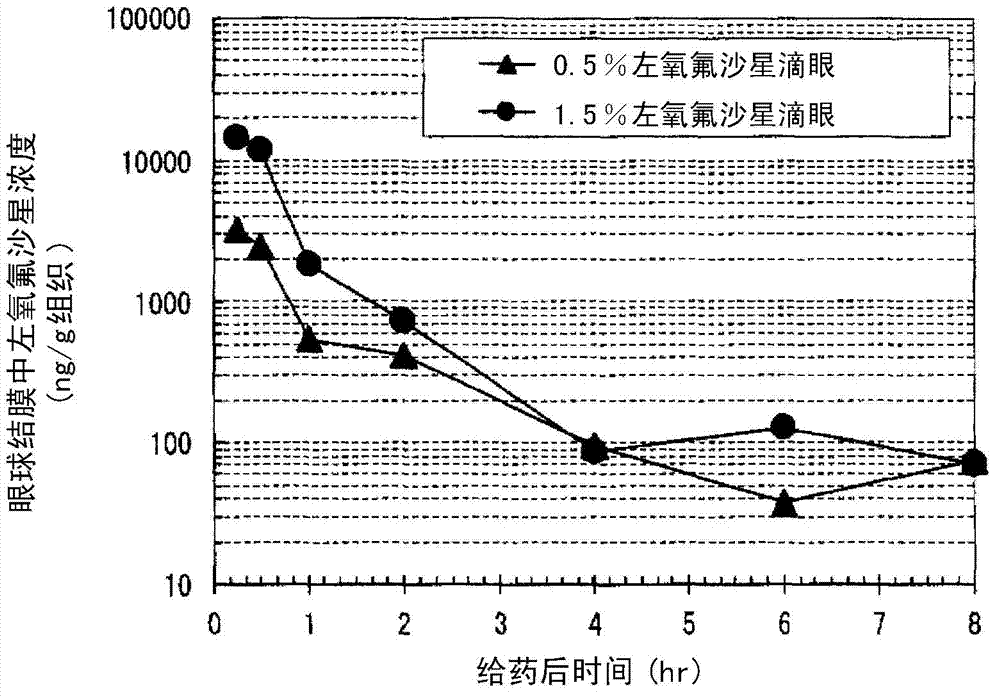
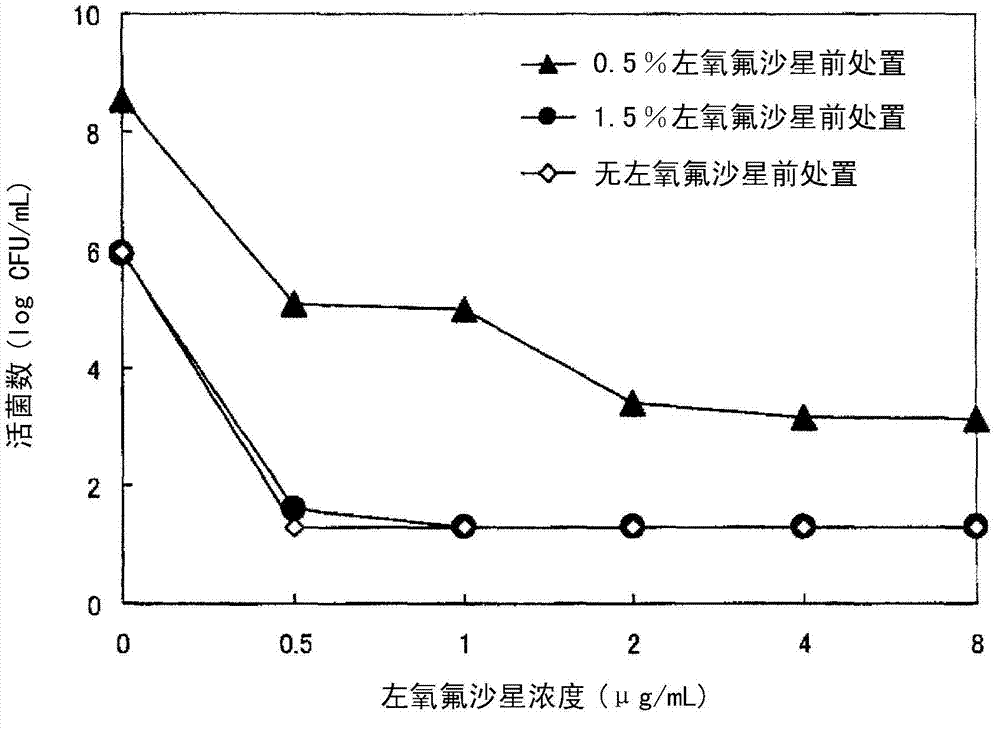
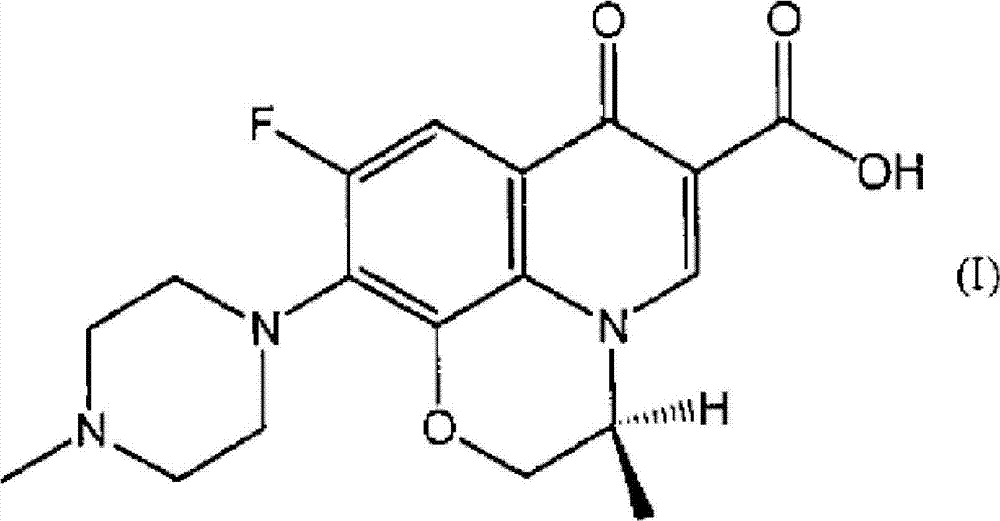
Eye drops for treating eye infection containing levofloxacin, salt thereof or solvate of same, method for treating eye infection, levofloxacin, salt thereof or solvate of same, and utilization thereof
A levofloxacin and solvate technology, which is applied to medical preparations containing active ingredients, anti-infective drugs, anti-inflammatory agents, etc., can solve the problems of drug-resistant bacteria without enlightenment, and achieve the effect of inhibiting drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0069] [Clinical Trials]
[0070] The clinical test shown below was carried out, for the 1.5% (w / v) levofloxacin eye drops of the current dosage 3 times a day and the 0.5% (w / v) levofloxacin eye drops of the previous usage 3 times a day The effect (efficacy and safety) of the liquid group on bacterial conjunctivitis was studied comparatively.
[0071] (Preparation method of eye drops)
[0072] 1.5% (w / v) levofloxacin eye drops and 0.5% (w / v) levofloxacin eye drops are made by dissolving levofloxacin 1 / 2 hydrate in water, adding isotonic agent (glycerin) and pH regulator, and using Prepared according to general techniques (pH: 6.1-6.9).
[0073] (test procedure)
[0074] According to the dosage of 1 drop each time and 3 times a day, 1.5% (w / v) levofloxacin eye drops or 0.5% (w / v) levofloxacin eye drops are used for patients with bacterial conjunctivitis. Use 14 days. Taking the start date of eye instillation, the 3rd day, 7th day and 14th day of eye instillation as the ref...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com