Compound capable of inhibiting zinc ion metalloproteinases
A metalloprotease and compound technology, applied in the preparation of organic compounds, active ingredients of heterocyclic compounds, active ingredients of carbohydrates, etc., can solve problems such as failure of clinical tests, impact on normal physiological functions, and low specificity of inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
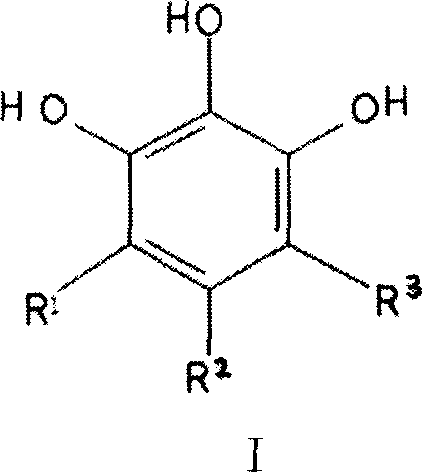
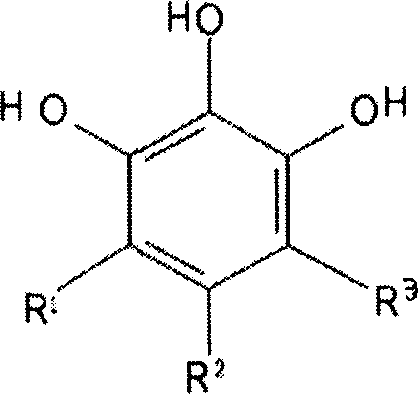
[0056] Example 1 1,2,3-trihydroxybenzene and its derivatives have inhibitory effect on zinc ion metalloprotease
[0057] Enzyme experiment steps:
[0058] The buffer system used is 50mM HEPES (pH7.5), with 0.2M NaCl, 10mM CaCl 2 , and 0.05% Brij-35, the substrates used are DQ-Gelatin and P126; measured at room temperature, the total reaction volume is 100ml. When adding inhibitors, the enzyme and inhibitor should be incubated in the buffer system for 30 minutes, and then added to the substrate assay. All detection instruments are FLX800 fluorescence microplate reader (Bio-Tek). For DQ-Gelatin, the excitation wavelength is 495nm, the emission wavelength is 515nm, and for P126, the excitation wavelength is 328nm, and the emission wavelength is 393nm.
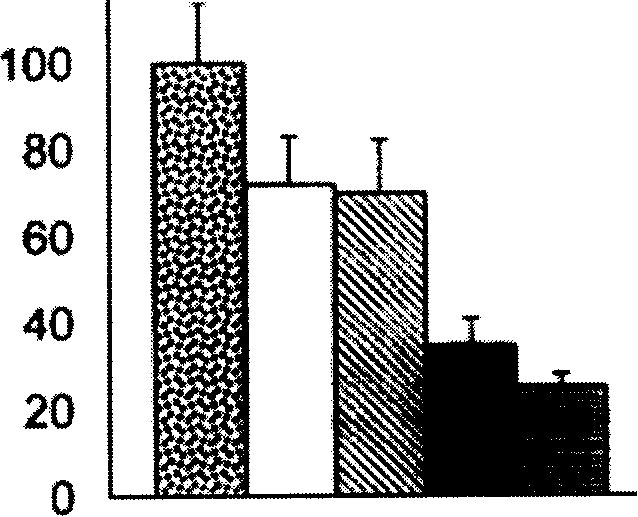
[0059] The measured reaction time is 8 minutes, and the slope of the line formed by the fluorescence value is taken as the velocity, and the one without inhibitor is recorded as V 0 , plus the inhibitor is V i , with V i / V ...
Embodiment 2
[0062] Example 2 Antitumor Experiment of Pyrogallic Acid
[0063] 1. Inhibition of adhesion and extension of HT1080 tumor cells by pyrogallic acid
[0064] Adhesion test:
[0065] After the HT1080 cells in the logarithmic growth phase were digested and resuspended, the cells were counted, and then counted with 10 3 The cell density per well was introduced into a 24-well plate, and a specified amount of pyrolytic acid was added to make the final concentration of the drug in each well be 0 μM, 2 μM, 5 μM, 10 μM, and 20 μM, and then 1 ml of 10% FBS was added to each well. After mixing the DMEM medium, put it in a 37°C incubator for about 30 minutes, take it out, and centrifuge it at 1000 rpm for 5 minutes with an orifice centrifuge, then suck off the supernatant, and observe the cells adhering to the bottom of the well in each well under an inverted microscope. number of cells.
[0066] Stretch test:
[0067] After the HT1080 cells in the logarithmic growth phase were digeste...
Embodiment 3
[0083] Example 3 Drug Treatment of Atherosclerosis with Specific Anti-MMP
[0084] 1. Atherosclerosis and MMPs
[0085] Atherosclerosis is the pathological basis of clinical syndromes such as acute myocardial infarction and unstable angina, and matrix metalloproteinases (MMPs) play an important role in cardiovascular remodeling by degrading extracellular matrix. The expression of MMPs is not only related to intimal thickening and plaque instability, but also related to the formation of restenotic lesions. The detected MMPs up-regulated in atherosclerosis include MMP-1, -2, -7, -9, -12. Many MMPs are related to matrix remodeling in atherosclerotic injury, such as MMP-1, -2, -3, -7, -9, -12, -13, -14. Therefore, the development of specific anti-MMP therapeutic drugs will help prevent the development of atherosclerosis, plaque rupture and restenosis.
[0086] 1. Atherosclerotic intima thickening: Atherosclerosis can be initiated by chemical or mechanical damage to the endothel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com