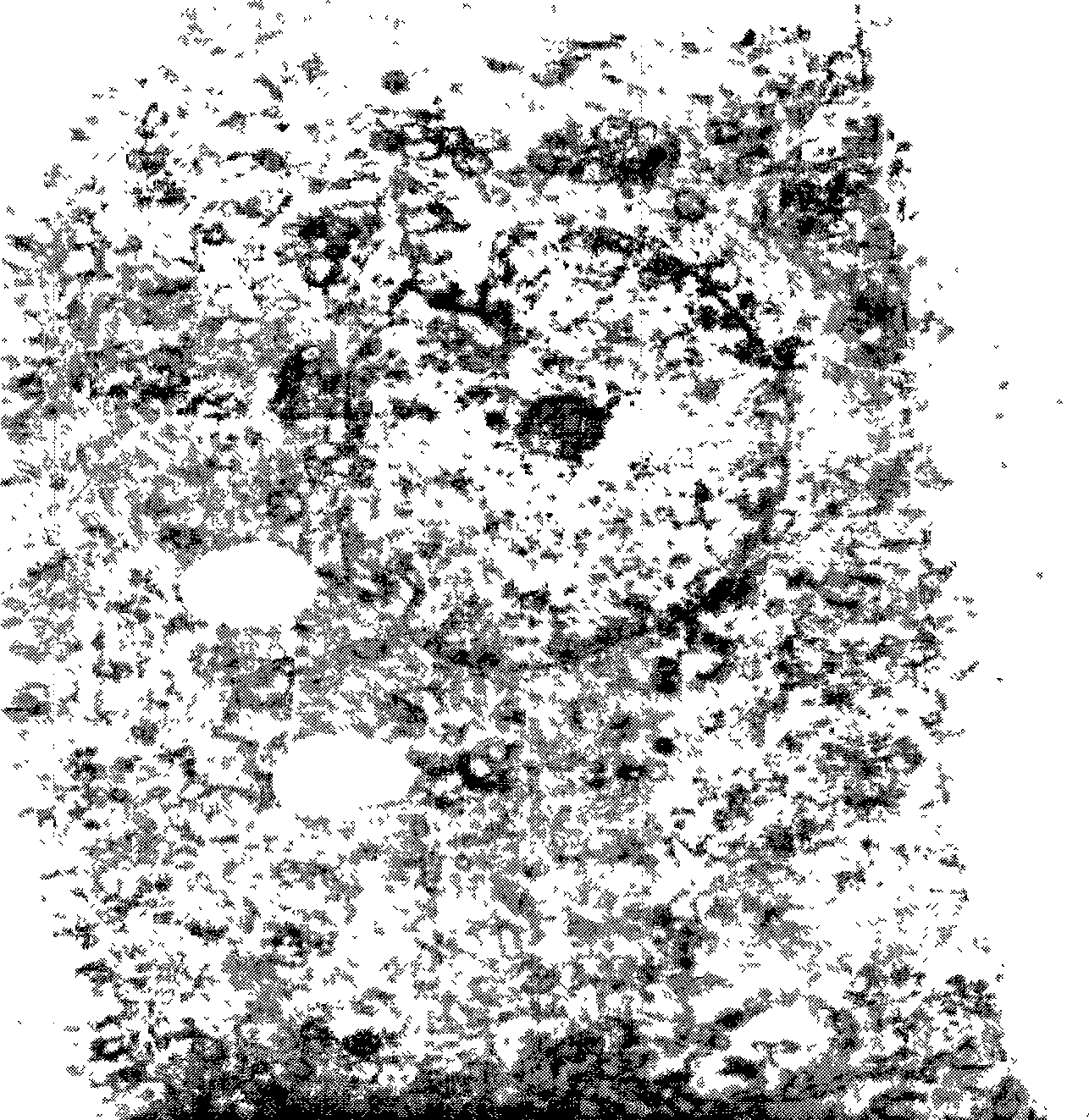Fresh amnion preservation fluid, fresh amnion preservation method and application thereof
A technology for preserving fluid and amniotic membrane, which is used in applications, preservation of human or animal bodies, animal husbandry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: the preparation of fresh amniotic membrane preservation solution
[0047] Precisely weigh the various substances in the formula, carry out in a sterile state according to the method and operating procedure of the medical tissue preservation solution, and pay attention to maintaining the pH value (6.8-7.4) of the preservation solution suitable for the physiological needs of the cells. May contain the following substances:
[0048] 1. 9.0 grams of DMEM medium
[0049] 2. Chondroitin Sulfate 10.0g
[0050] 3. Sodium hyaluronate 0.5g
[0051] 4.HEPES 4.0g
[0052] 5. Low molecular weight dextran 10.0g
[0053] 6. Gentamicin 1.0ml (40,000 units / ml)
[0054] 7. Dexamethasone 24.0 mg
[0055] 8. Non-essential amino acids 10.0ml
[0056] 9. Reduced glutathione 0.50g
[0057] 10. Fetal bovine serum 20.0ml
[0058] 11. Add water for injection to 1000 ml.
[0059] Electron micrograph after above-mentioned solution preservation 30 days, as figure 1 As shown...
Embodiment 2
[0061] Precisely weigh the various substances in the formula, carry out in a sterile state according to the method and operating procedure of the medical tissue preservation solution, and pay attention to maintaining the pH value (6.8-7.4) of the preservation solution suitable for the physiological needs of the cells. It can also be this content ratio:
[0062] 1. 12.0 grams of DMEM medium
[0063] 2. Chondroitin Sulfate 25.0g
[0064] 3. Sodium Hyaluronate 1.0g
[0065] 4.HEPES 5.0g
[0066] 5. Low molecular weight dextran 15.0g
[0067] 6. Gentamicin 3.0ml (40,000 units / ml)
[0068] 7. Dexamethasone 30.0 mg
[0069] 8. Non-essential amino acids 15.0ml
[0070] 9. Reduced glutathione 0.95g
[0071] 10. Fetal bovine serum 50.0ml
[0072] 11. Add water for injection to 1000ml
Embodiment 3
[0074] Precisely weigh the various substances in the formula, carry out in a sterile state according to the method and operating procedure of the medical tissue preservation solution, and pay attention to maintaining the pH value (6.8-7.4) of the preservation solution suitable for the physiological needs of the cells. It can also be formulated with the following contents:
[0075] 1. 12.0 grams of DMEM medium
[0076] 2. Chondroitin Sulfate 25.0g
[0077] 3. Sodium Hyaluronate 1.0g
[0078] 4.HEPES 5.0g
[0079] 5. Low molecular weight dextran 15.0g
[0080] 6. (Antibiotic) Gentamicin 3.0ml (40,000 units / ml)
[0081] 7. Dexamethasone 30.0 mg
[0082] 8. Non-essential amino acids 15.0ml
[0083] 9. Reduced glutathione 0.95g
[0084] 10. Add water for injection to 1000 ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com