New Proteases Able to Hydrolyze Gluten Peptides and Proteins at Acidic PH, from the Actinomycete Actinoallomurus
a technology of endopeptides and proteases, which is applied in the direction of peptide/protein ingredients, immunological disorders, drug compositions, etc., can solve the problems of very difficult diet and strong patient demands, and achieve the effects of reducing the levels of toxic gluten oligopeptides, preventing symptoms, and effectively producing protein hydrolyzates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
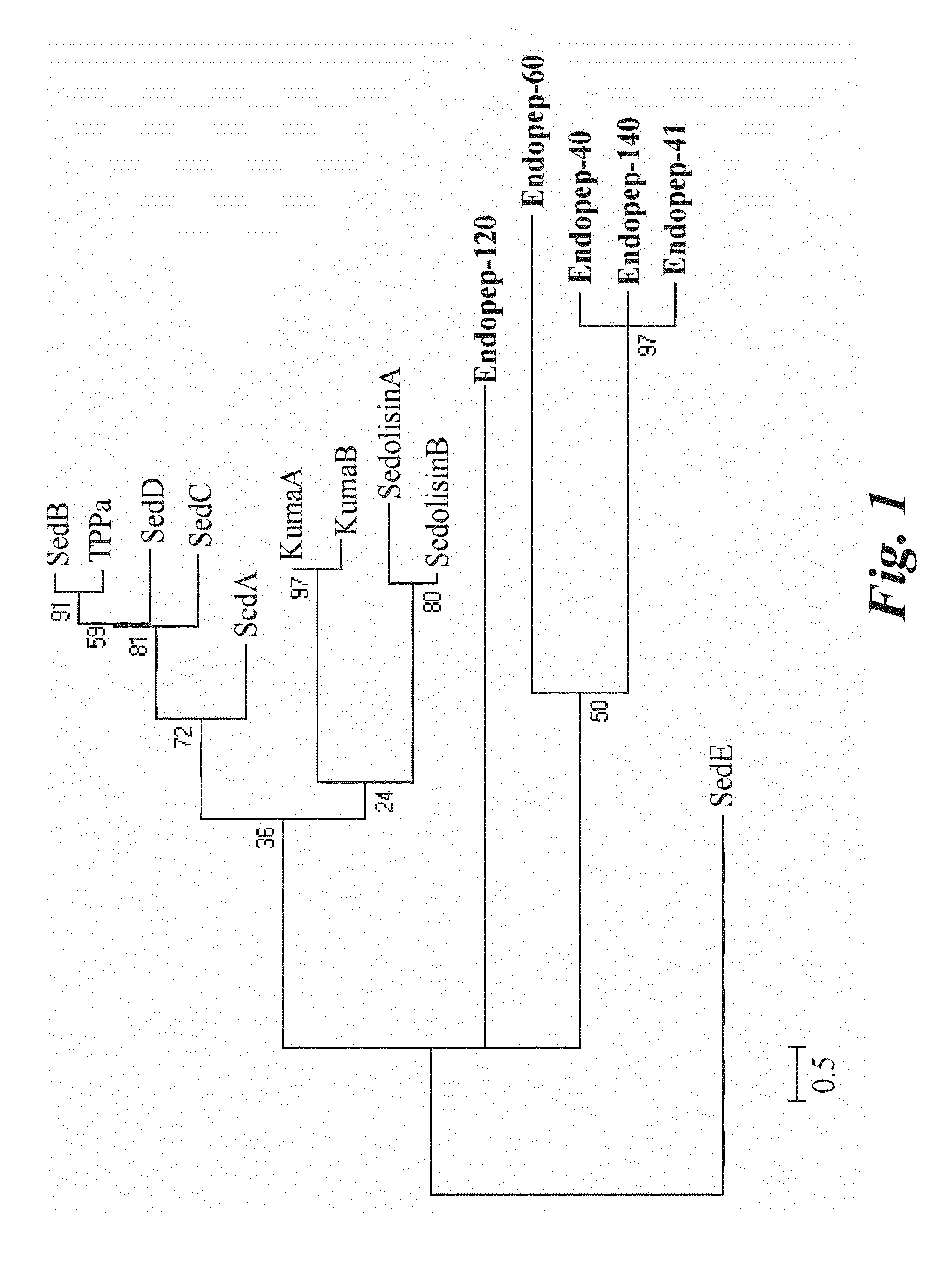
Actinoallomurus sp. DSM 24988 Endopeptidases Identification and Characterization
1.1 Strain Cultivation and Protein Production
[0106]The Actinoallomurus strain used according to this invention derives from the Applicants strain collection.
[0107]Actinoallomurus sp. DSM 24988 was maintained on ISP2 agar medium (Shirling and Gottlieb, 1966) acidified at pH 5.5 with HCl. The microbial content of one plate was scraped and inoculated into one 50 ml Erlenmeyer flask containing 15 ml of medium AF5 which is composed of: (g / l) dextrose 20, yeast extract 2, soybean meal 8, NaC11 and MES 10. Medium was prepared in distilled water and pH adjusted to 5.5 prior to sterilization at 121° C. for 20 min. The inoculated flask was grown at 28° C., on a rotary shaker operating at 200 rpm. After 5-6 days incubation, 5% of culture was inoculated into a second series of 500 ml Erlenmeyer flasks containing 100 ml of the same fermentation medium. Protein production was performed in flasks incubated for 15 days ...
example 2
Endopeptidase Production in Recombinant Host Cells
2.1 Strains and Plasmids.
[0133]Actinoallomurus sp. DSM 24988 was used in this study. All plasmid subcloning experiments were performed in E. coli DH10B (Invitrogen, Carlsbad, Calif.) using the plasmid pTZ57R / T (Fermentas, UAB, Lithuania).
[0134]Escherichia coli B121(DE3) Star(Novagen Italia, Podenzano, PC) was used to produce heterologous (recombinant) peptidases.
2.2 Recombinant Protease Production
[0135]Recombinant Actinoallomurus proteases were produced and purified from Escherichia coli B121(DE3) Star used as the expression system.
[0136]To construct E. coli strains producing Endopep-140 and Endopep-40 nucleotide sequences of genes were amplified using the following set of primers:
Fendopep-140SEQ ID NO: 125′-AAAAAGCTTCAGCTACAGGTGTGGTCGG-3′Rendopep-140SEQ ID NO: 135′-AAAAAAACATATGCCCGATCTTCCCACCC-3′Fendopep-40SEQ ID NO: 145′-AAAAAGCTTCAGAAGGCTCCGGTGCC-3′Rendopep-40SEQ ID NO: 155′-AAAAAAACATATGTCACGACGCGTGACCG-3′
[0137]The PCR products ...
example 3
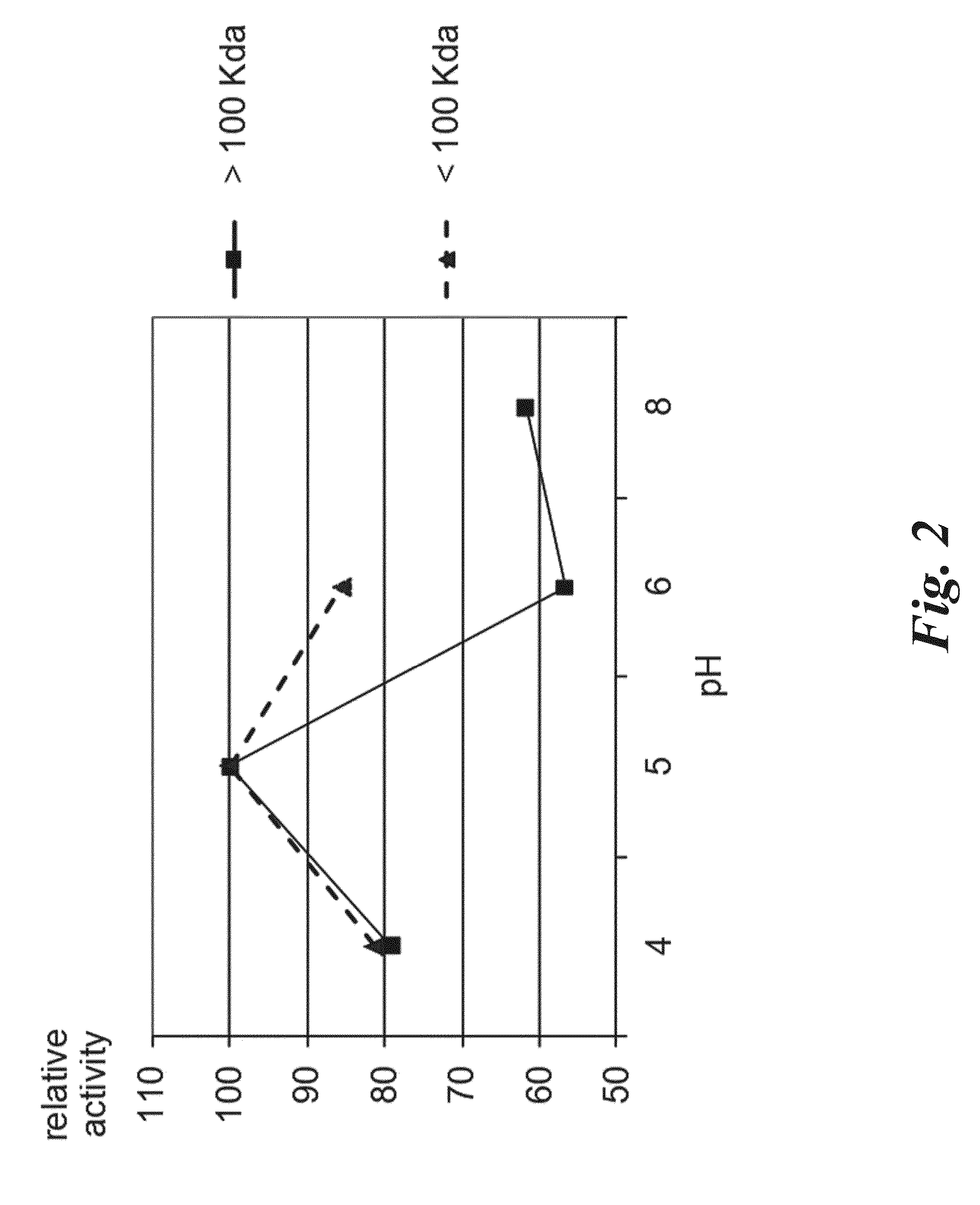
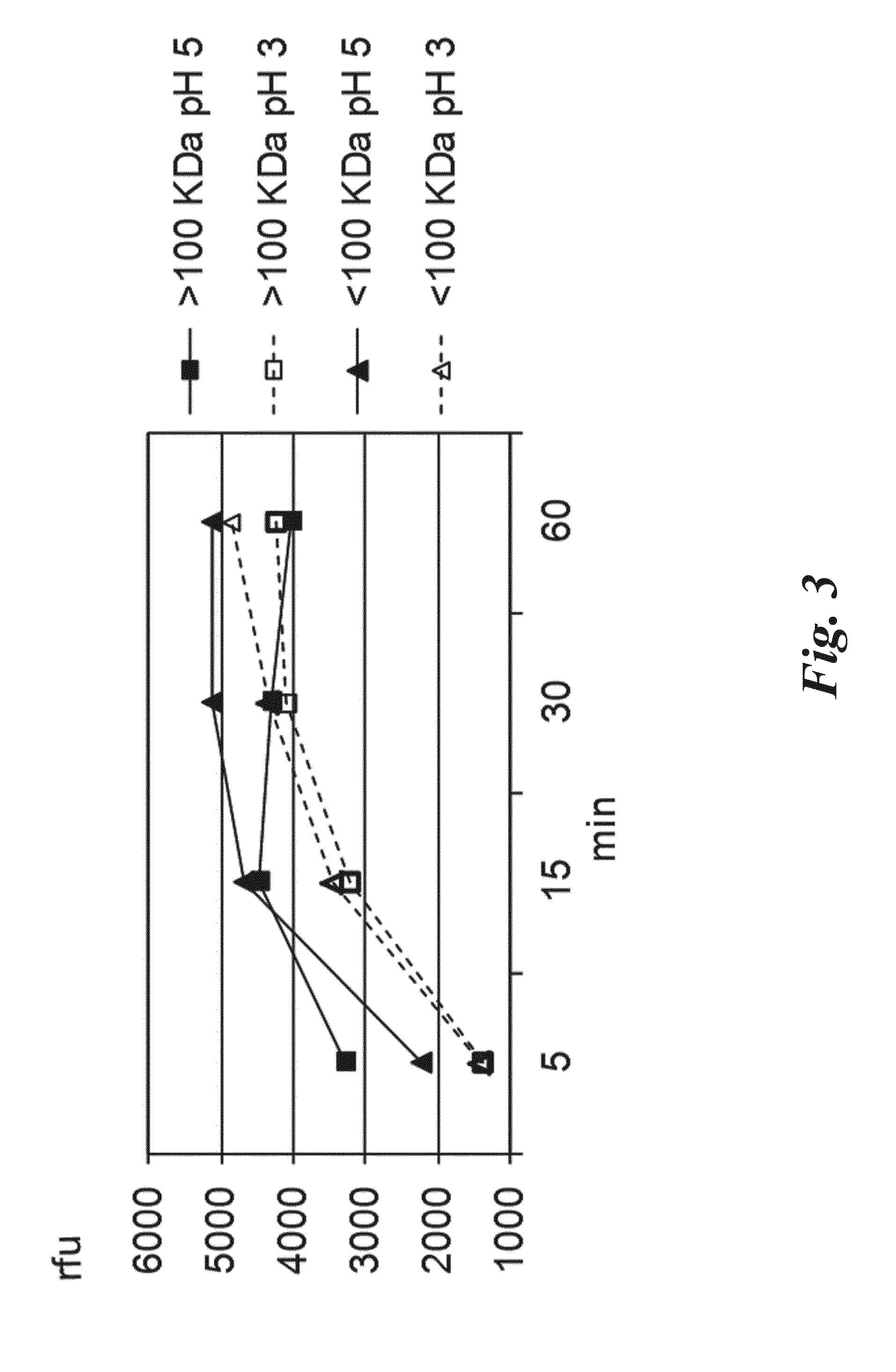
Endopeptidase Biological Activity
3.1 Degradation of 33-Mer Toxic Peptide of Gliadin at Acidic pH
[0141]A solution of 33-mer immunotoxic peptide of gliadin (50 μM) was incubated at 37° C. for up to 2 hours in the presence of 4 μU of endopep-140 or 2 μU endopep-40 in presence or absence of pepsin 1 mg / ml. The reaction was carried out in acetic acid 20 mM pH 3, total volume of 300 μl. The reaction was monitored at different times from 0 to 120 min (t0, t3, t15, t30, t60 and t120 min) at 37° C. Disappearance of the 33-mer peptide and appearance of degradation products was monitored by HPLC-MS analysis. 50 μl aliquots were taken and the enzyme activity was stopped with 50 μl H2O: CH3CN 50:50 (+0.1% formic acid). The samples were submitted to HPLC-MS, analyzed on LTQ-XL mass spectrometer (Thermo Fisher Scientific, San Jose, Calif., USA). The HPLC-MS profiles obtained with endopep-140, endopep-140 and pepsin, pepsin alone are reported in FIGS. 5, 6, 7 respectively. The disappearance of the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com