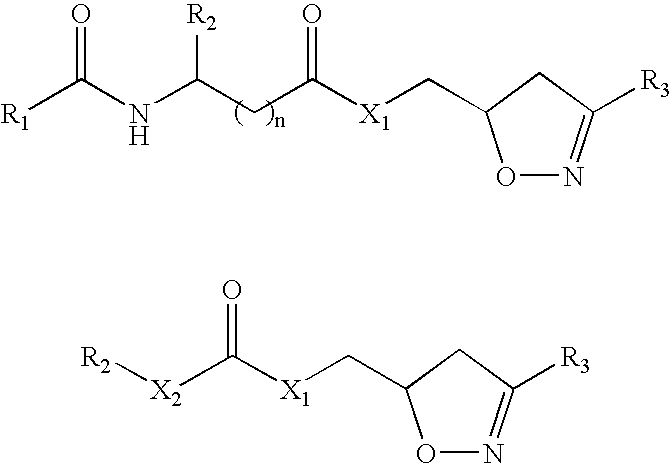
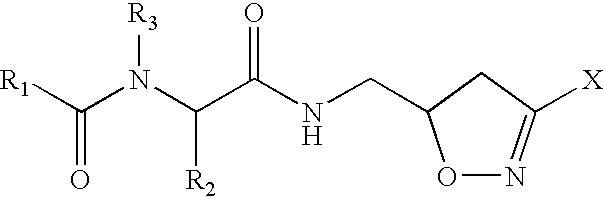
Drug therapy for celiac sprue
a celiac sprue and drug therapy technology, applied in the field of celiac sprue drug therapy, can solve the problems of no good treatment for the disease, few patients respond poorly or not at all, and erroneous diagnosis of eczema
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of dihydroxyisoxazole Containing tTGase INHIBITORS
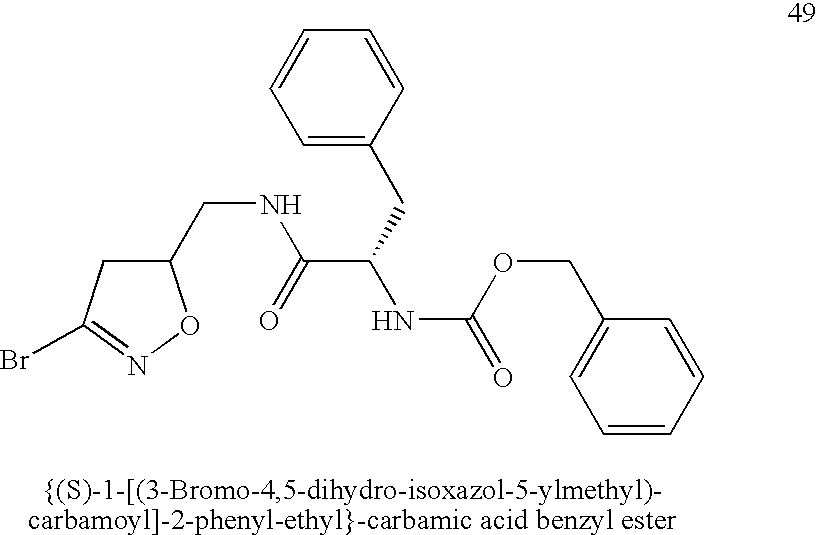
[0065] Synthesis of {(S)-1-[(3-Bromo-4,5-dihydro-isoxazol-5-ylmethyl)-carbamoyl]-2-phenyl-ethyl}-carbamic acid benzyl ester (n=0, X═NH, R1=BnO, R2═(S)-Bn, R3═Br) (49). N-Cbz-L-Phe (0.30 g, 1.0 mmol) and HOBt (0.15 g, 1.1 eq) were dissolved in 2 mL DMF. 3-Bromo-5-aminomethyl-4,5-dihydroisoazole (0.18 g, 1.0 eq), prepared following a reported procedure (Rohloff et al. (1992) Tetrahedron Lett. 33(22):3113-3116), was added to the solution cooled in an ice bath followed by EDCI (0.23 g, 1.2 eq). The ice bath was removed and the stirring was continued overnight. The solution was diluted with ethyl acetate and washed with sat. NaHCO3 solution and brine. The organic layer was dried over MgSO4 and filtered. The solvent was removed by evaporation and the residue was purified by SiO2 chromatography to give the title compound as a white solid (0.24 g, 52%).
[0066]1H NMR (CDCl3, 200 MHz): δ=7.34-7.26 (m, 8H), 7.17 (d, 2H, J=7.6 Hz), 6....
example 2
Synthesis of Dioxoindole Containing tTGase Inhibitors
[0111] Synthesis of 2,3-Dioxo-2,3-dihydro-1H-indole-5-sulfonic acid propylamide. 2,3-Dioxo-2,3-dihydro-1H-indole-5-sulfonyl chloride (0.10 g, 0.41 mmol), prepared by the reaction of the sodium salt of 5-isatinsulfonic acid with POCl3, was dissolved in 5 mL THF. This solution was cooled in an ice bath and DIEA (0.14 mL, 2.0 eq) was added slowly, followed by n-propylamine (35 uL, 1.0 eq). Stirring was continued for 40 min and the solution was diluted with ethyl acetate and washed with brine. The organic layer was dried over Na2SO4 and the solvent was removed by evaporation. The residue was purified by SiO2 chromatography to give the title compound (65 mg, 60%).
[0112]1H NMR (CD3CN, 400 MHz): δ=9.17 (br, 1H), 8.02 (d, 1H, J=8.0 Hz), 7.93 (s, 1H), 7.13 (d, 1H, J=8.0 Hz), 5.62-5.58 (m, 1H), 2.85-2.80 (m, 2H), 1.48-1.42 (m, 2H), 0.85 (t, 3H, J=7.2 Hz)
[0113] MS (ESI): m / z=−267.1 [M−H]−
[0114] Synthesis of 2,3-Dioxo-2,3-dihydro-1H-indole...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight loss | aaaaa | aaaaa |
| stability | aaaaa | aaaaa |
| oxidative stress | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com