Preparation of full-length human antibody
A humanized antibody, antibody technology, applied in the field of preparation of fully humanized antibody
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
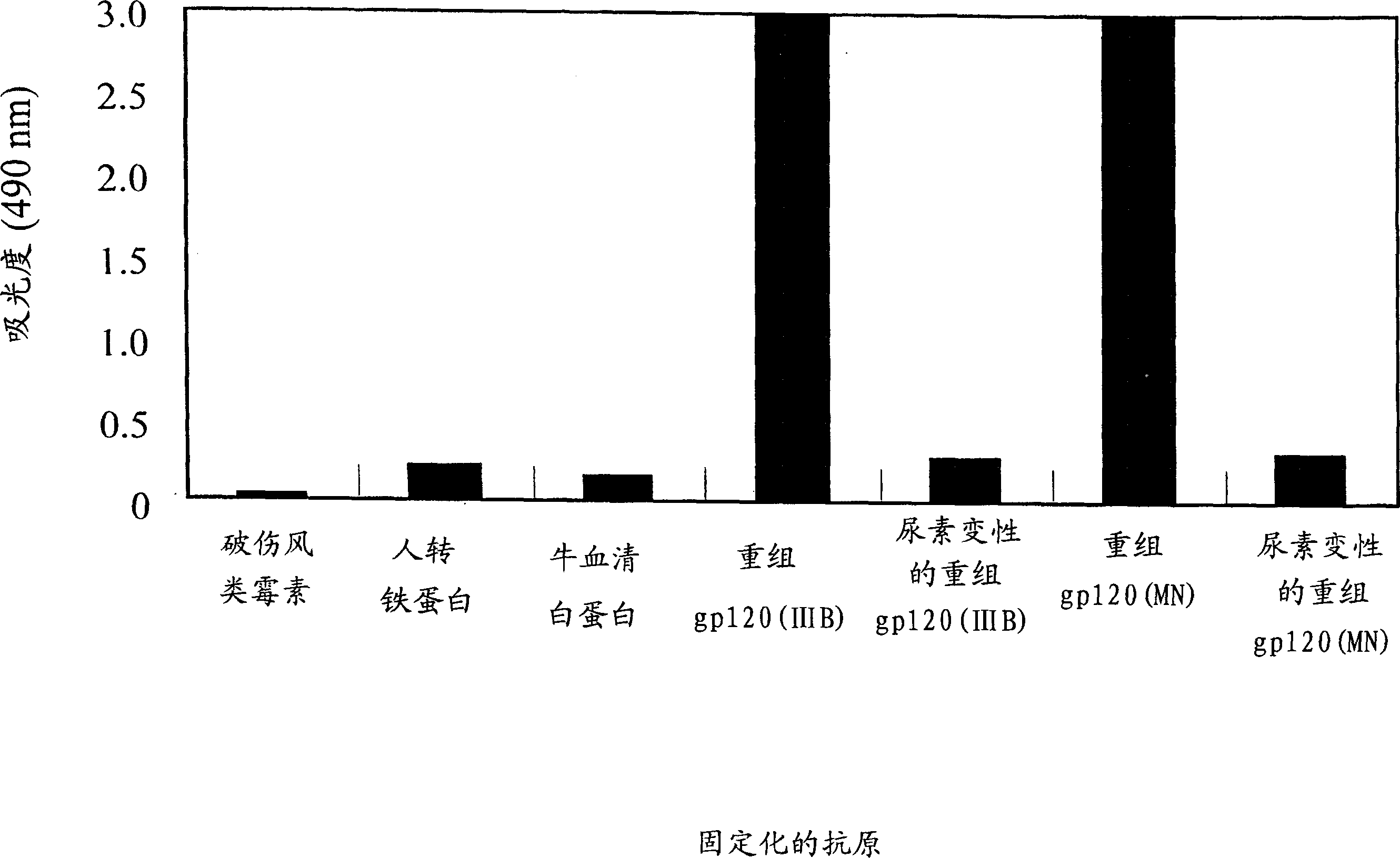
[0083] The present invention provides a method for producing fully humanized antibodies capable of recognizing a predetermined antigen without relying on human donors who have been exposed to the antigen. To achieve this, lymphocytes from natural human donors are immunized with the antigen of interest in vitro, and then antibody-producing cells against the antigen are identified and screened. Since lymphocytes immunize in vitro rather than in vivo, it is possible to regulate the antigen or antigen fragment recognized by the antibody. A preferred antigen is the HIV gp120 protein, particularly the binding site of the gp120 co-receptor molecule.
[0084] Unless otherwise stated, before further elaborating the present invention, the terms used in this application are defined as follows.
[0085] A "fully humanized antibody" refers to an antibody that contains only human sequences. The antibody is preferably a monoclonal antibody. A "naive human donor" is a person who has never b...
Embodiment 1
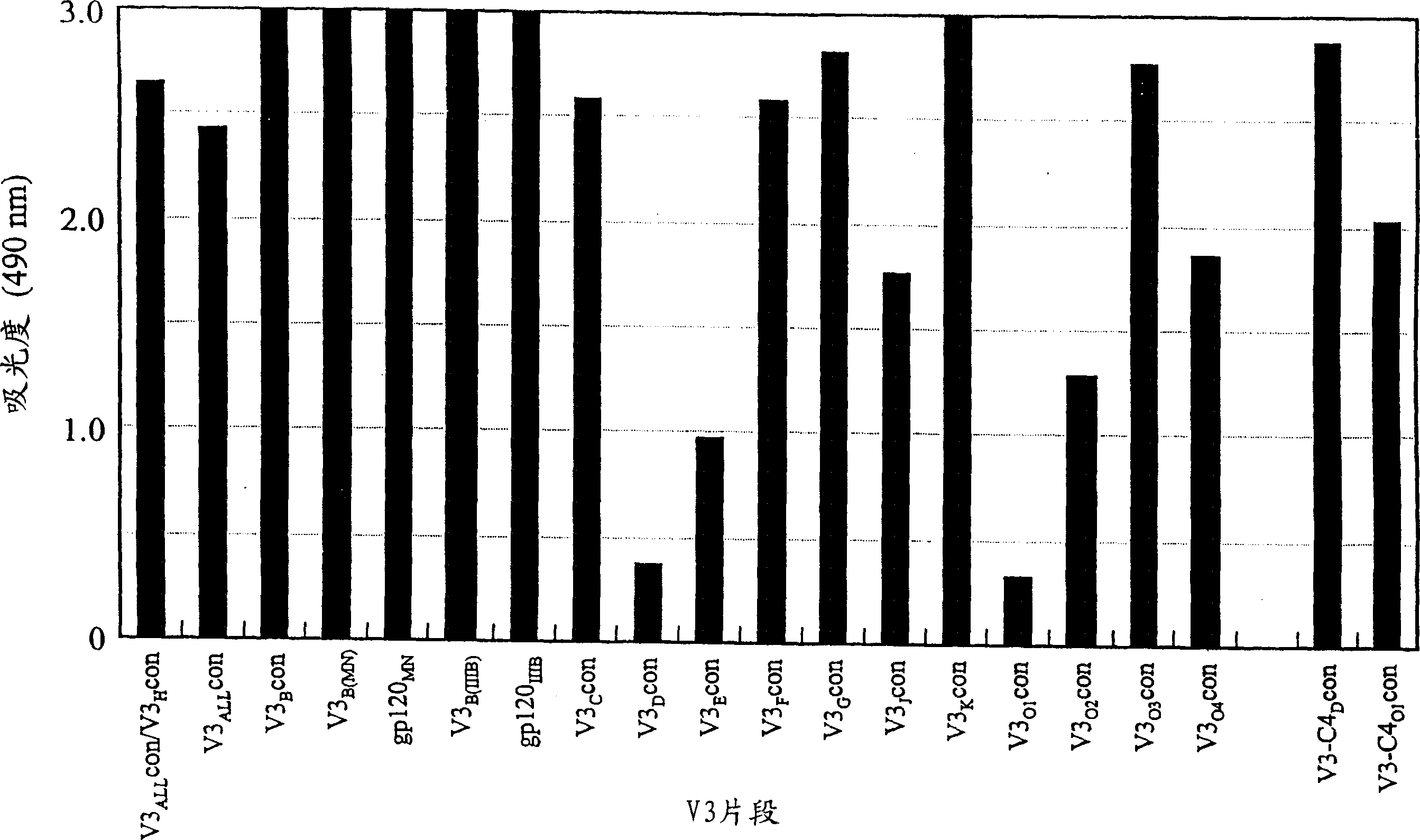
[0177] Preparation of peptide antigens
[0178] Synthetic peptides corresponding to HIV-1 gp120 V3 loop are listed in Table 1.
[0179] Table 1
[0180] Synthetic Peptide Amino Acid Sequence of Synthetic Peptide in HIV-1 gp120 V3 Co-receptor Molecular Binding Region
[0181] HIV-1 strain / subtype amino acid sequence and sequence comparison SEQ ID NO.
[0182] V3 ALL con HIV-1 consensus sequence N’- R K S I H I . . G P G Q A F Y A T -C’ 2
[0183] V3 A con Subtype A consensus sequence N’- - - - V R - . . - - - - - - - - - - -C’ 3
[0184] V3 B con Subtype B consensus sequence N’- - - - - - - . . - - - R - - - T - -C’ 4
[0185] V3 B(MN) MN(B subtype) N'- - - R - - - . . - - - R - - - T - -C' 5
[0186] V3 B(IIIB) IIIB(B subtype) N'- - - - - R - Q R - - - R - - V - C' 6
[0187] V3 C con Subtype C consensus sequence N’- - - - - R - . . - - - - T - - - - -C’ 7
[0188] V3 D con Subtype D consensus seq...
Embodiment 2
[0203] CD8 + and CD56 + Depletion of cell populations results in increased antigen-responsive immune cells
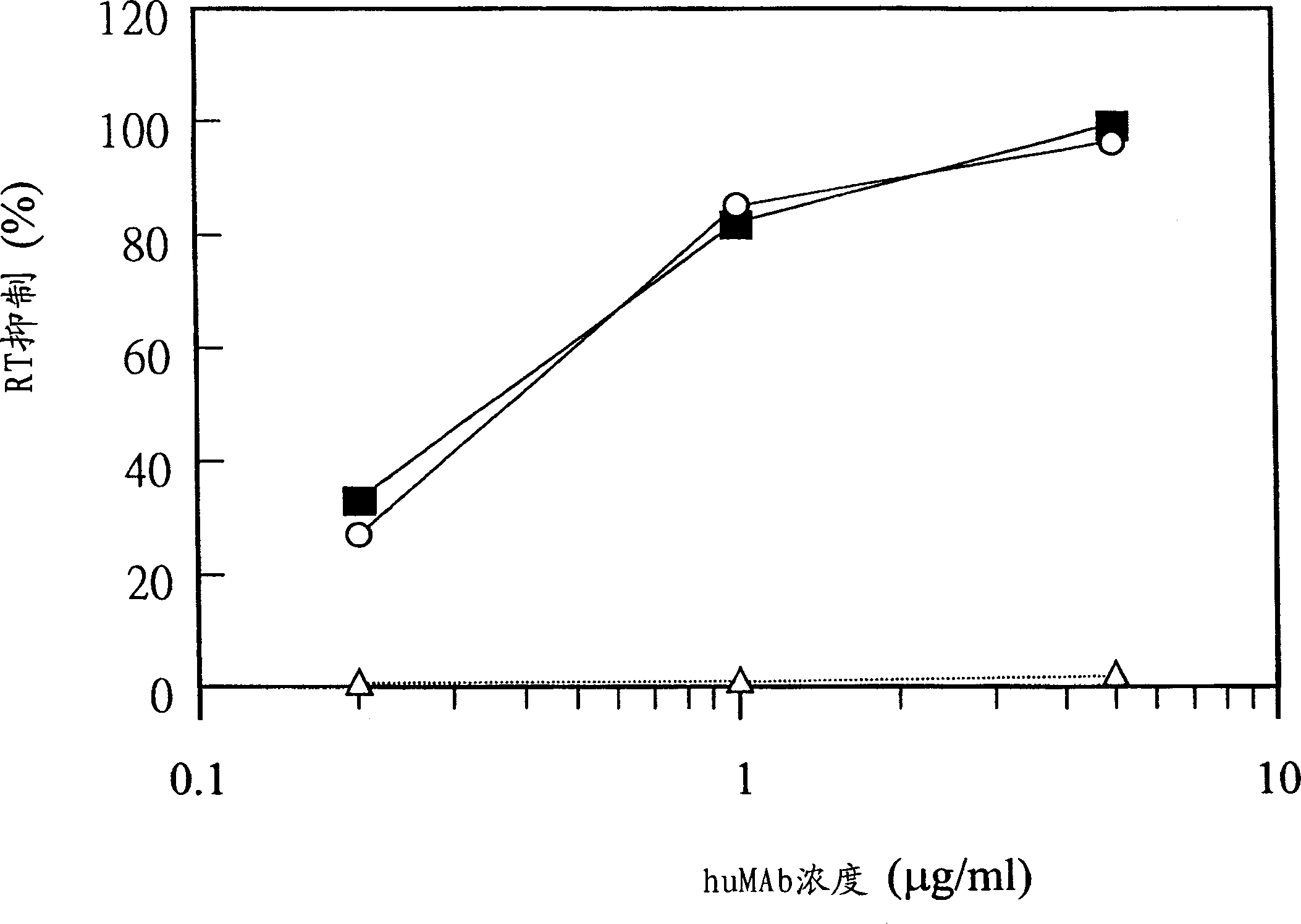
[0204] Because the removal of cytotoxic or suppressive cell populations plays an integral role in the overall immune response to antigens in vitro (Ohlin et al., 1989; 1992), various methods for removing cells were compared in in vitro immunological studies. Effects of cytotoxic agents. These reagents include LeuLeuOMe, anti-CD8 antibody used in cell magnetization removal, anti-CD56 antibody used in cell magnetization removal, and combination of anti-CD8 antibody and anti-CD56 antibody used in cell magnetization removal. Thus, peripheral blood mononuclear cells (PBMC S ) were treated with each reagent, or no reagent was added as a blank control (nil). Four PBMCs from different donors were tested. In vitro immunizations were performed as described in the "Materials and Methods" section, including primary and secondary immunizations with the peptide antigen TT-V3B(MN...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com