Freeze-dried human lymphocyte CD4 surface antigen quality control material and preparation method thereof
A lymphocyte and surface antigen technology, applied in the field of immunological detection, can solve the problems of harsh storage and transportation conditions, the microspheres easily exceed the collection range, and the storage period is short, saving transportation and storage costs, and improving the stability of antigen expression. and reproducibility of cell properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The application of the present invention provides a method for preparing a freeze-dried human lymphocyte CD4 surface antigen quality control product, as follows:
[0033] 1. Collect human peripheral blood cells through venous blood collection, spread the cell suspension on the sucrose solution, and centrifuge to make cells of different densities aggregate, and collect human peripheral lymphocytes;
[0034] 2. Rinse with phosphate buffered saline containing serum;
[0035] 3. Add anti-human CD4 antibody, protect from light, incubate at 18°C for 20 minutes, collect cells after centrifugation, resuspend cells in phosphate buffer, elute unbound excess antibody, and harvest antibody-labeled cells by centrifugation;
[0036] 4. To harvest the cells, follow 10 3 Cells / mL concentration, add fixative, mix thoroughly by shaking, and fix at 6°C for 0.5 hours in the dark;
[0037] 5. Elute the fixative, use a flow cytometer, and count the number of positive cells. According to ...
Embodiment 2
[0042] The application of the present invention provides a method for preparing a freeze-dried human lymphocyte CD4 surface antigen quality control product, as follows:
[0043] 1. After washing the buffy coat (Buffy coat), collect human peripheral blood cells, spread the cell suspension on commercial human lymphocyte separation medium, and centrifuge to make cells of different densities aggregate, and collect human peripheral lymphocytes;
[0044] 2. Rinse with Hank's buffer;
[0045] 3. Add anti-human CD4 antibody, protect from light, incubate at 30°C for 30 minutes, and collect cells after centrifugation. Re-suspend cells in Hank's solution, elute unbound excess antibody, and harvest antibody-labeled cells by centrifugation;
[0046] 4. Follow 10 3 Cells / mL concentration, add fixative solution, mix thoroughly by pipetting, and fix at 25°C for 36 hours in the dark;
[0047] 5. Elute the fixative, use a flow cytometer, and count the number of positive cells. According to t...
experiment example 1
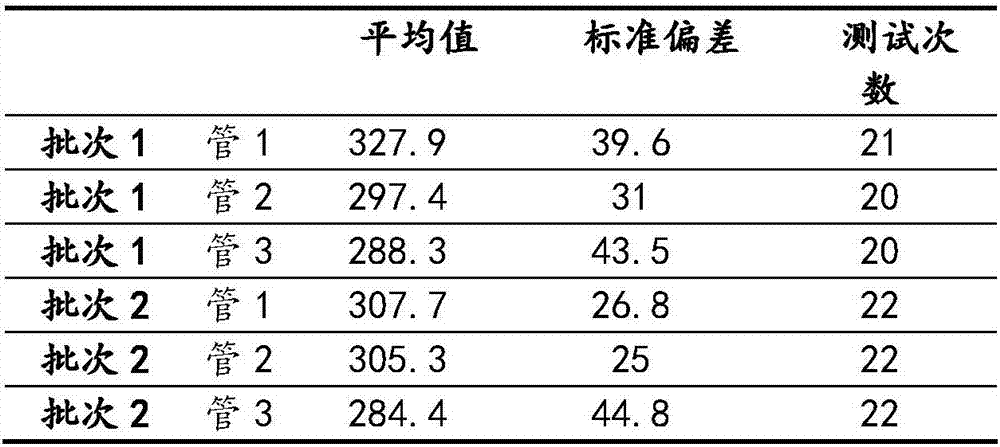
[0052] Take samples from two different batches, 3 bottles from each batch. Add 1ml of ultrapure water to each bottle and place it at 15-30°C for 5-10 minutes to fully absorb and redissolve the freeze-dried cells. After fully mixing, each bottle of cell suspension was evenly distributed to 20-22 flow-type sample loading tubes in the amount of 50 μl / tube, and 150 μl of phosphate buffer was added to each sample loading tube. Detection was performed on BDFACSCalibur and BC Cytomics FC 500 flow cytometers, respectively. The results are shown in the table below:
[0053] Table 1
[0054]
[0055] The results show:
[0056] 1. The cells prepared by this method maintain complete cell morphology and cell contents, and are similar or identical to fresh cells;
[0057] 2. The cells prepared by this method maintain the expression of surface antigens, and there is no loss of target antigens;
[0058] 3. The positive cells prepared by this method have the same detection results on d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com