Placental hematopoietic stem cell and preparation method thereof and placental hematopoietic stem cell injection
A technology for hematopoietic stem cells and placenta, applied in the field of placental hematopoietic stem cells and their preparation, and injection of placental hematopoietic stem cells, can solve the problems of affecting vitality, cell damage, rough grinding method, etc., and achieve the effect of increasing quantity and vitality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: Preparation method of the present invention prepares placental hematopoietic stem cells
[0034] Placenta with negative test results for infectious diseases such as hepatitis, syphilis, and AIDS and without obstetrical complications were selected, and the informed consent of the puerpera was obtained and signed an informed consent form before the operation. A sterile polystyrene storage bottle was prepared before the operation as a transport bottle, which contained commercially available cell culture medium, and was sent to the preparation place within 12 hours at 4°C after collection to separate and prepare placental hematopoietic stem cells.
[0035]In a sterile ultra-clean bench, take 1-2ml of placenta collection liquid for sterility testing, then remove the placental capsule, cut the placental tissue, put the chopped placental tissue into a beaker and wash it repeatedly with normal saline for 1-3 times , wash away the blood remaining on the surface of ...
Embodiment 2
[0043] Embodiment 2: the contrast of cell quantity and vitality
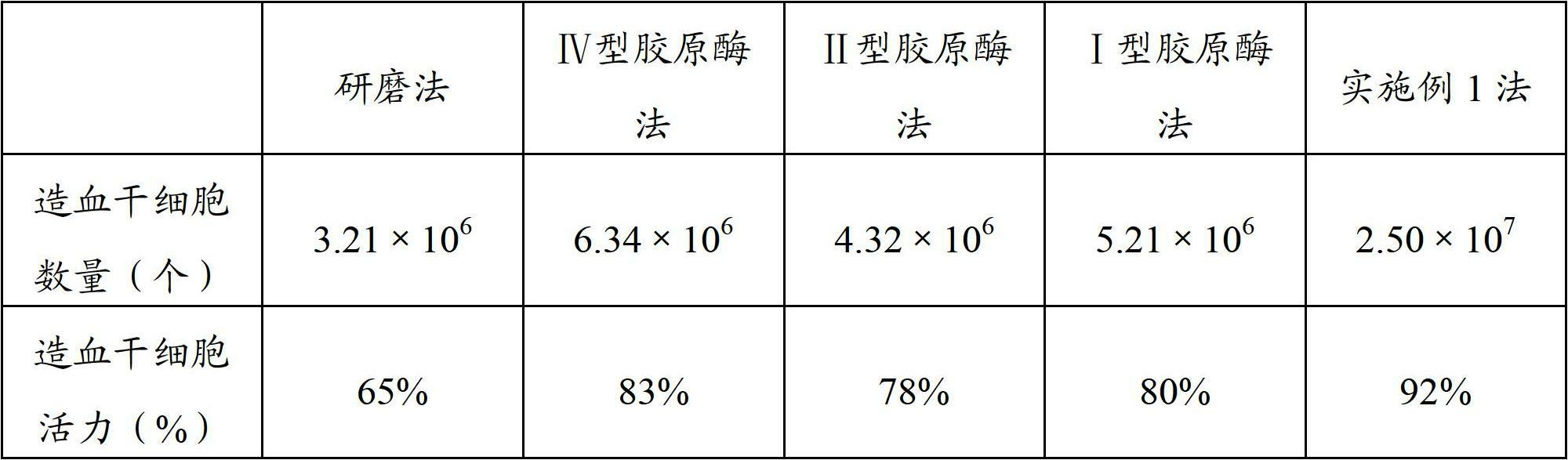
[0044] The placenta with the same source and volume as in Example 1 was used to prepare placental hematopoietic stem cells by using the existing grinding method and single collagenase method, and the steps involving resuspension, washing, centrifugation, etc. were all consistent with those in Example 1. In the single collagenase method, the amount and the mass percentage of collagenase of the single collagenase digestion solution adopted are all the same as the amount and the collagenase mass percentage of the corresponding collagenase digestion solution in Example 1, and finally these several methods are prepared The number and viability of the placental hematopoietic stem cells were detected, and the results are shown in Table 1.
[0045] Table 1 Cell number and viability
[0046]
[0047] It can be seen from the above table that the number of placental hematopoietic stem cells prepared by multiple collage...
Embodiment 3
[0048] Embodiment 3: Preparation method of the present invention prepares placental hematopoietic stem cells
[0049] Placenta with negative test results for infectious diseases such as hepatitis, syphilis, and AIDS and without obstetrical complications were selected, and the informed consent of the puerpera was obtained and signed an informed consent form before the operation. A sterile polystyrene storage bottle was prepared before the operation as a transport bottle, which contained commercially available cell culture medium, and was sent to the preparation place within 12 hours at 4°C after collection to separate and prepare placental hematopoietic stem cells.
[0050] In a sterile ultra-clean bench, take 1-2ml of placenta collection liquid for sterility testing, then remove the placental capsule, cut the placental tissue, put the chopped placental tissue into a beaker and wash it repeatedly with normal saline for 1-3 times , wash away the blood remaining on the surface of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com