AFP and GM-CSF dual-gene co-expression recombinant vector as well as preparation method and application of recombinant vector
A technology of GM-CSF and recombinant vector, which is applied in the field of recombinant vector and its preparation, can solve the problems of lack of immunotherapy targeting, immune regulation, and small anti-tumor ability, achieve good liver cancer immunotherapy effect, and promote T cell Immune response, enhancing the effect of immune regulation ability of the body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Obtaining AFP gene fragments containing specific enzyme cleavage sites
[0047] 1. Primer design
[0048] According to the nucleotide sequence of the AFP gene (as shown in SEQ ID NO: 1 in the sequence listing) and the expected insertion multiple cloning site on the pIRES2-EGFP plasmid vector, design specific primers as follows:
[0049] AFP upstream primer (as shown in SEQ ID NO:4 in the sequence listing):
[0050] 5'-GC AGATCT ATGAAGTGGGTGGAA-3' (the underlined part is the sequence of the Bgl II restriction site),
[0051] AFP downstream primer (as shown in SEQ ID NO:5 in the sequence listing):
[0052] 5'-TT GAATTC TTAAACTCCCAAAAGCAGC-3' (the underlined part is the sequence of EcoR I restriction site).
[0053] 2. Obtain cDNA template
[0054] RNA was extracted from human liver cancer cell HepG2 by TRIzon method (TRIzon total RNA extraction kit was purchased from Beijing Kangwei Century Biotechnology Co., Ltd., product number is CW0580), and reverse...
Embodiment 2
[0061] Example 2: Construction of pIRES2-AFP-EGFP recombinant vector
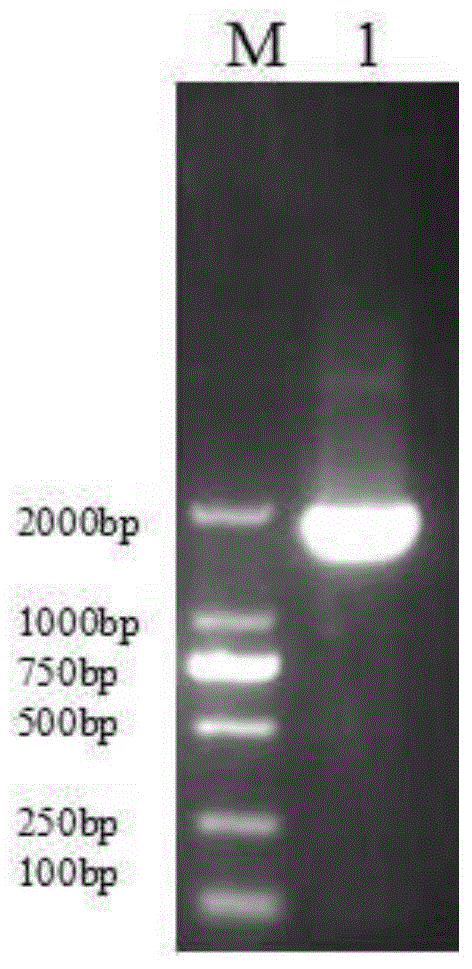
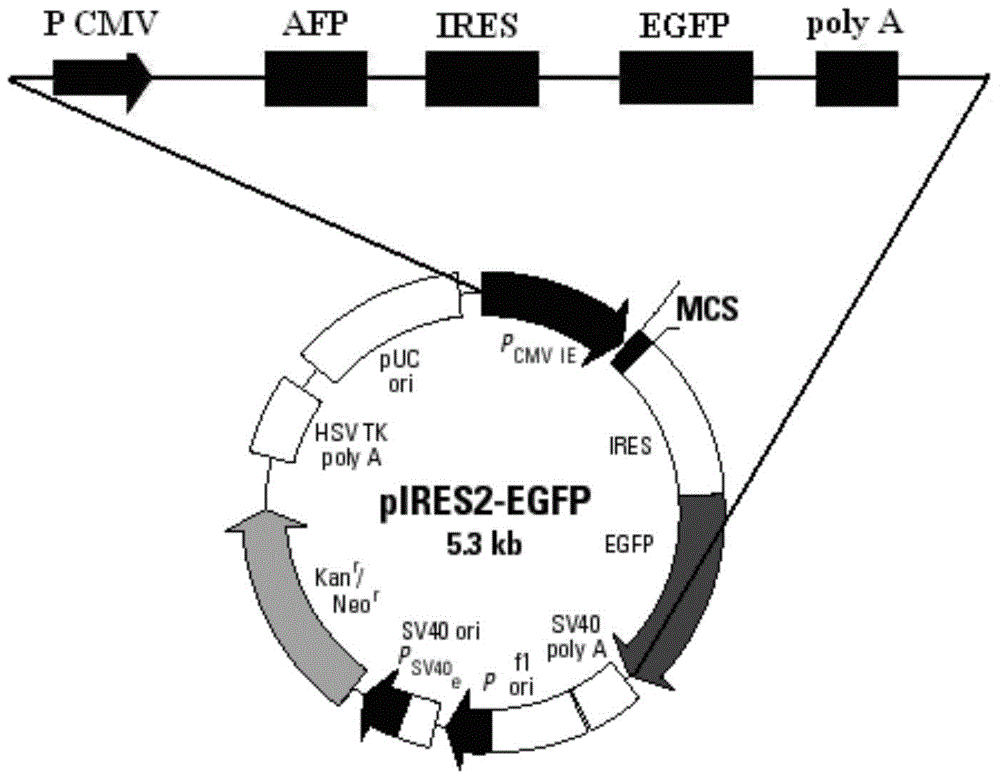
[0062] Using restriction endonucleases Bgl II and EcoR I, digest the pIRES2-EGFP plasmid (the multiple cloning site of the plasmid contains Bgl II, EcoR I restriction sites) and the AFP gene fragment obtained in Example 1, respectively, to obtain Linearized pIRES2-EGFP vector and digested AFP gene sequence after enzyme digestion; use T4 DNA ligase system for ligation reaction, incubate at 22°C for 30 minutes, and then inactivate at 70°C for 5 minutes to construct pIRES2-AFP -EGFP recombinant vector (such as image 3 shown).
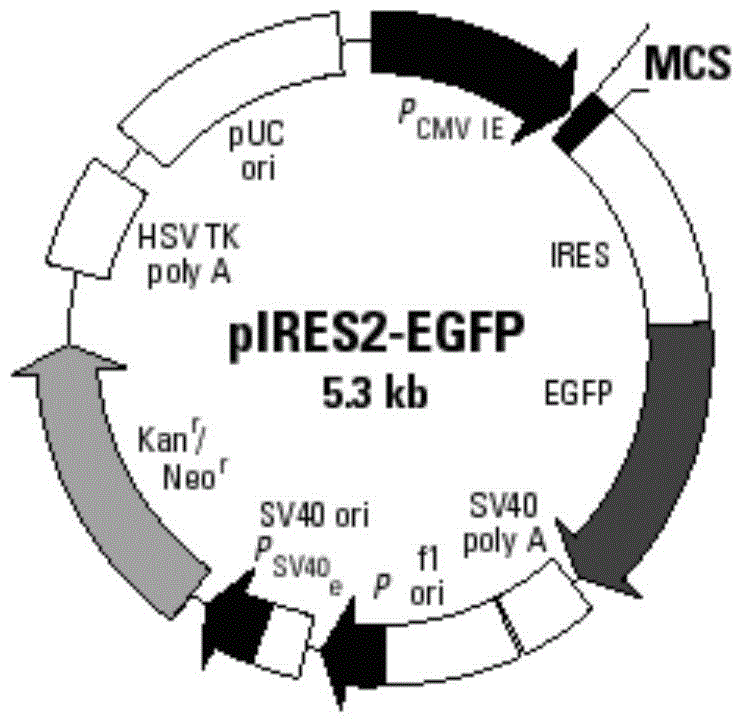
[0063] Structural features of the pIRES2-EGFP plasmid (eg figure 2 Shown) It can be seen that after the AFP gene is inserted into the multiple cloning site of the pIRES2-EGFP plasmid, it is located upstream of the self-sequence IRES of the plasmid vector (such as image 3 shown), that is, the AFP and EGFP sequences under the same promoter were expressed separately.
[0064] 1. Dou...
Embodiment 3
[0090] Example 3: Double PCR method to obtain GM-CSF gene fragments with restriction endonuclease cohesive ends
[0091] According to the GM-CSF gene sequence and the expected insertion multiple cloning site on the pIRES2-EGFP plasmid vector, design two pairs of primers with different lengths and sticky ends with restriction endonucleases; reverse transcribe with RNA extracted from CIK cells The cDNA of cDNA was used as a template, and the above two pairs of primers were used for PCR amplification to obtain two PCR amplification products; the two PCR amplification products were mixed and then denatured and annealed sequentially to obtain four GM-CSF gene fragments, wherein Both GM-CSF gene fragments have restriction endonuclease cohesive ends, which allow directional ligation of the GM-CSF gene fragments into the desired polyclonal of the plasmid vector without the need for restriction endonuclease digestion site.
[0092] Compared with the traditional PCR product cloning met...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com