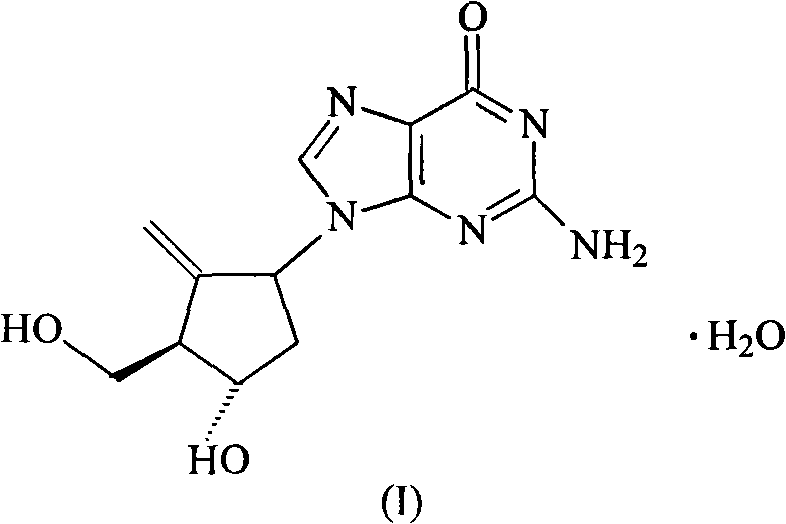
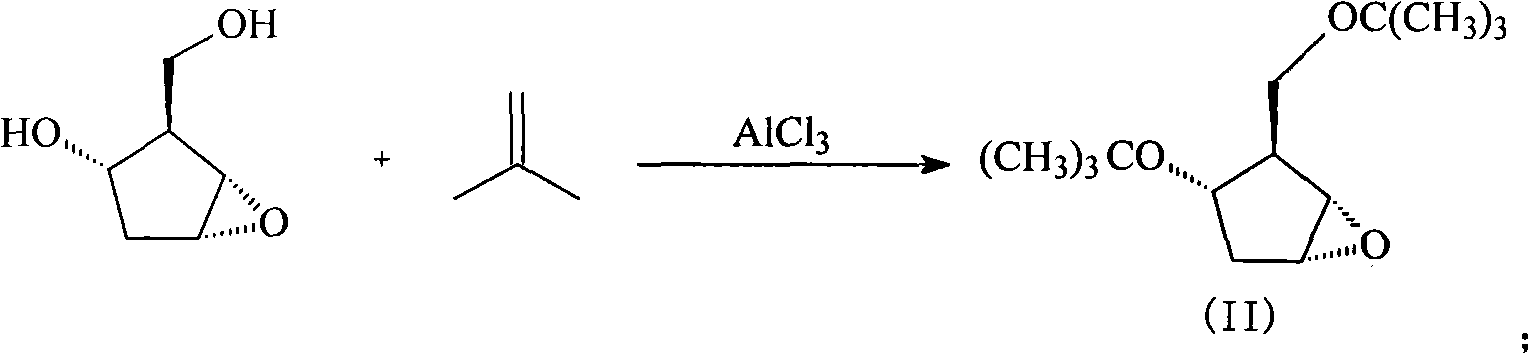Entecavir compound prepared in novel method
A technology of entecavir and compounds, which is applied in the field of entecavir compounds, can solve the problems of high import prices, low product purity, and increased costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The synthesis of embodiment 1 entecavir
[0038] (1) Synthesis of intermediate (II)
[0039] Add 130g (1mol) [1S-(1α, 2α, 3β, 5α)-2-hydroxymethyl]-6-oxabicyclo[3.1.0]hexan-3-ol into a 3L reaction flask, then add 134g (1.2mol) of isobutylene and 1000ml of anhydrous acetone, stirred, then added 133g (1mol) of aluminum chloride, stirred at room temperature for 15 hours, then evaporated the solvent under reduced pressure, and dissolved the residue with 1500ml of dichloromethane , and then washed with water, washed with saturated aqueous sodium chloride solution, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 220 g of intermediate (II) product with a yield of 91%.
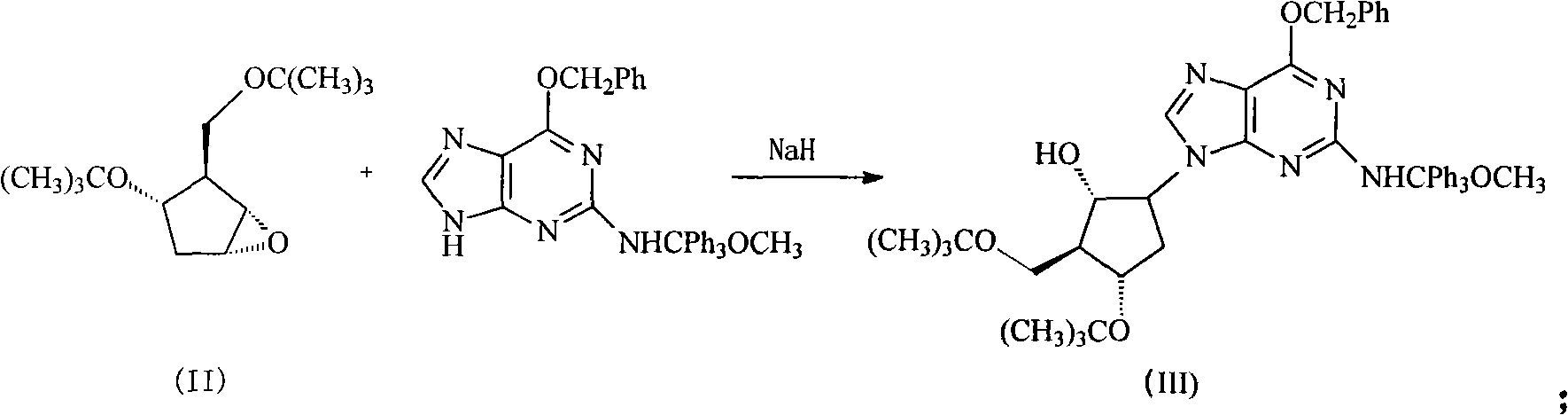
[0040] (2) Synthesis of intermediate (III)
[0041] Under nitrogen protection, 51g (0.1mol) of [2-[[(4-methoxyphenyl) diphenylmethyl] amino]-6-benzyloxy-9H-purine and 100g of sodium hydride (Content 60%) was dissolved in the DMF of 500ml, stirred and reacted at room temp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com