A colorimetric immunoassay for the detection of tumor markers for non-diagnostic purposes
A tumor marker and immunoassay technology, applied in the field of colorimetric immunoassay, can solve the problems of short reaction time, expensive equipment, complicated operation, etc., and achieve the effect of simple operation, fast response and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
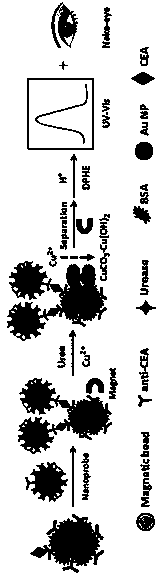
[0021] Example 1 A colorimetric immunoassay method for detecting the tumor marker CEA for non-diagnostic purposes, comprising the following steps:
[0022] (1) Preparation of functionalized magnetic beads
[0023] Take 4μL of 25mg / mL carboxylated magnetic beads, under the action of an external magnetic field, wash three times with 50mM tris(hydroxymethyl)aminomethane-hydrochloric acid buffered saline solution, pH=7.4, and add to the washed magnetic beads Mix 200 μL of 0.2M 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) and 0.2M N-hydroxysuccinimide (NHS), shake at room temperature React for 30 minutes to activate the carboxylic acid groups on the surface of the magnetic beads. After the activated magnetic beads were washed three times with 50mM Tris-HCl solution, they were redispersed in 400μL of 2-(N-morpholine)B with a concentration of 50mM and pH=6.0. Add 10 μL of carcinoembryonic antigen CEA-coated antibody at a concentration of 1 mg / mL to the sulfonic a...
Embodiment 2
[0026] The detection of CEA content in the embodiment 2 standard solution
[0027]Take 40 μL of the prepared functionalized magnetic beads and place them in 6 sample tubes, dilute the CEA standard solution with a concentration of 1 mg / mL with 50 mM Tris-HCl solution at pH=7.4 to a concentration gradient of 0.001, 0.01, 0.1, 1, 10, and 100ng / mL CEA standard stock solution, put 100 μL of the above six different concentrations of CEA standard stock solution in the above six sample tubes in turn, and incubate at 37°C for 20 minutes with shaking. Under the action of an external magnetic field, use Tris-HCl solution containing 0.05% (w / v) Tween at a concentration of 50 mM, pH = 7.4 and Tris-HCl solution were washed alternately, and then 100 μL of urease functionalized gold nanoprobe was added to each sample tube respectively, Shake and incubate at 37°C for 20 minutes, wash the product three times under the action of an external magnetic field, and then add urea with a concentration ...
Embodiment 3
[0030] Example 3 Detection of CEA content in human serum
[0031] Get three clinical serum samples, carry out 5 parallel experiments respectively for each sample, the processing method of sample is the same as above-mentioned standard solution, see the following table 2 through the concentration of CEA in the tested sample, use the present embodiment method of above-mentioned three clinical serum samples Compared with the measurement results of the Beckman chemiluminescence immunoassay analyzer, the results are shown in Table 2 below.
[0032] Table 2 Comparison of the measurement results between this example and the Beckman Chemiluminescent Immunoassay Analyzer
[0033]
[0034] As can be seen from Table 2 above, the relative standard deviation (RSD) of the test results in this example is 1.8-5.6%, and the relative error is -3.7-3.1%, indicating that the immunoassay method of this example has high repeatability and stability. Compared the test results of this embodiment ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com