Improvements on tissue sealant for use in non-compressible hemorrhage
A tissue and pressure technology, applied in the field of four-component solutions, can solve problems such as inability to rinse or immediately eliminate, adverse reactions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
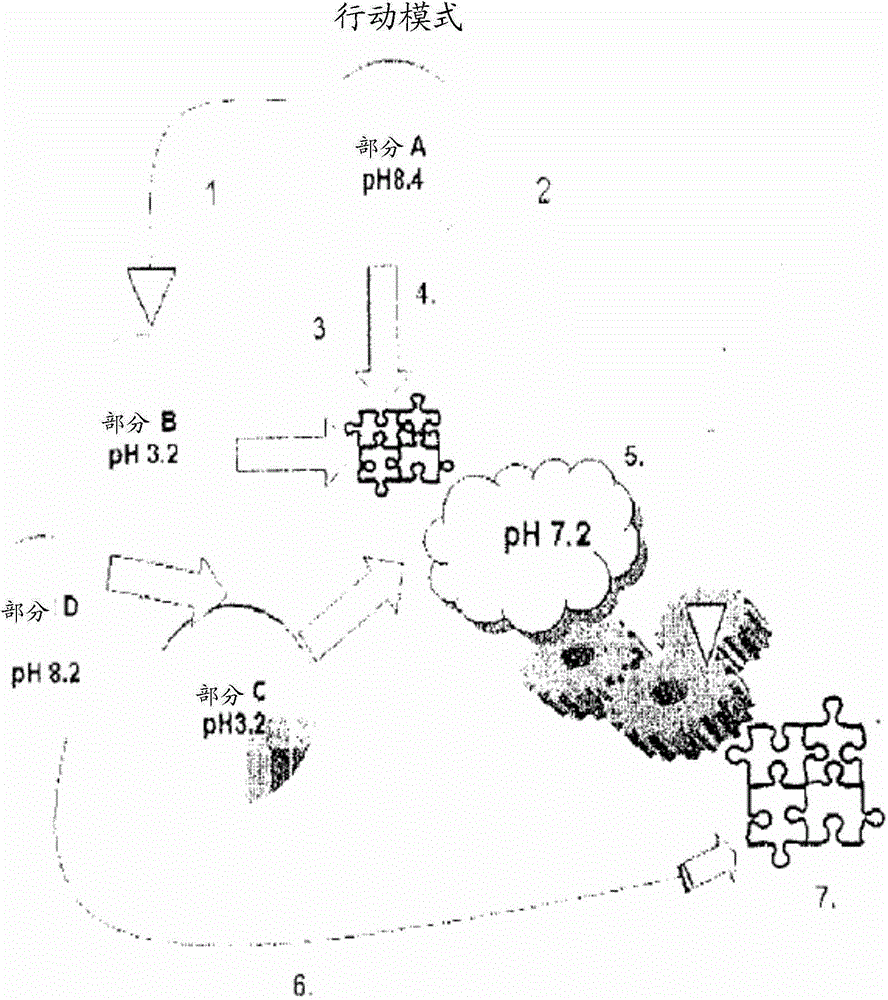
[0069] Step 1. Preparation of neutral A:
[0070] ZnCl in the micromolar range
[0071] MgSO in the millimolar range 4
[0072] Radix Fin Cold Water Fish Gel
[0073] sucrose
[0074] polyvinylpyrrolidone
[0075] h 2 O,
[0076] Stir all contents until homogeneous, and then neutralize the solution to pH 7.1 with NaOH
[0077] Step 2. Preparation of Final Solution A
[0078] Neutral A" (above)
[0079] Carrageenan, Type 2 or alpha-galactosidase degraded very small amounts of carrageenan, or alginate sulfate or hyaluronate or hyaluronic acid
[0080] NaHCO 3
[0081] human serum albumin
[0082] All components are stirred, thereby producing a suspension, which is then homogenized.
[0083] Step 3) Preparation of Solution B
[0084] NaH 2 PO4
[0085] tris hydroxymethyl amino methane
[0086] Carbomer 934
[0087] Step 4) Preparation of Solution C
[0088] A 90-120 mg / ml solution of fibrin monomer in 0.125% ice-cold AcOH (pH 3.4) was prepared by dialysis.
[...
example
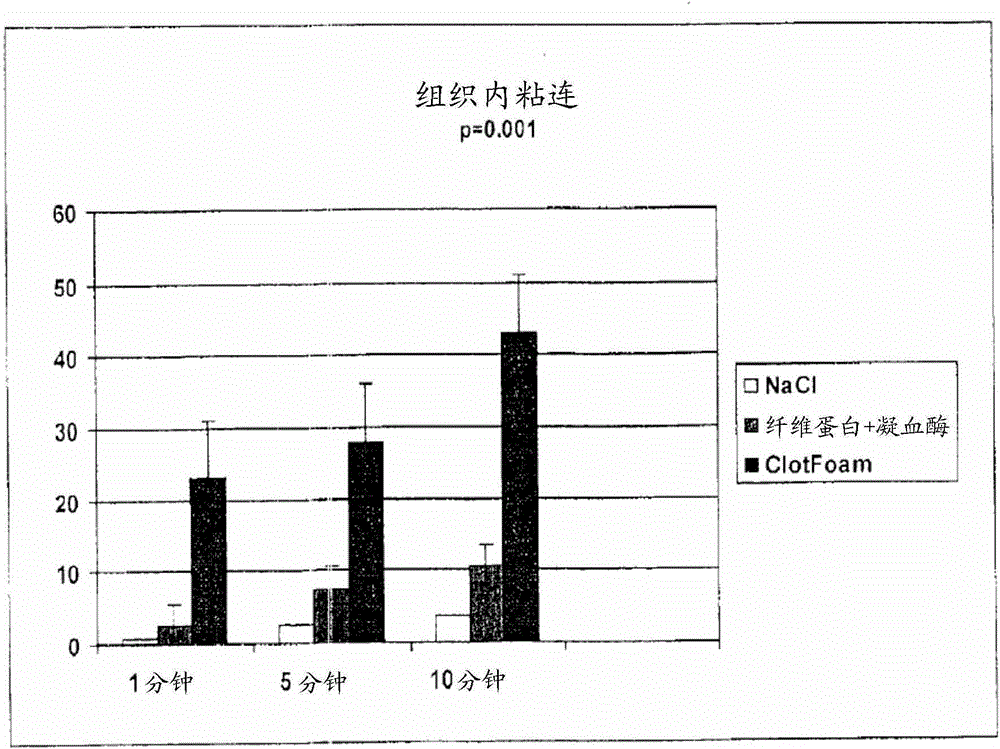
[0097] 1. Adhesive and viscoelastic properties: The adhesive properties to vital human tissues and the kinetics of gel polymerization have been tested in in vitro and ex vivo studies.
[0098] 1.1. Adhesive properties
[0099] Adhesion and tensile measurements (intra-tissue adhesion and clot strength) were performed in liver tissue of Sprague-Dawley rats. The liver was chosen because it is the organ most frequently injured in abdominal trauma, followed by the spleen.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com